Megan Bowers, Durham University
There is growing interest in the use of natural compounds such as phytochemicals in medicine. Turmeric is known to possess many health benefits and has been used for centuries for culinary and traditional uses. Its most potent phytochemical, curcumin, has been subject to extensive research and exhibits biological activities, including anti-cancer activities. However, it has not been approved for therapeutic use due to poor bioavailability. Recently, efforts have turned to improving its bioavailability, such as the use of adjuvant phytochemicals. Turmeric contains a variety of phytochemicals, many of which also possess anti-cancer activities themselves. In this review, the evidence for the superior bioavailability and chemopreventative activities of turmeric compared to curcumin alone are discussed, and mechanisms that may underlie these observations are highlighted. More research should be done to uncover the interactions of the phytochemicals within turmeric. Most research has focused on the use of curcumin and other phytochemicals as potential adjuvants in cancer treatment. However, a large proportion of cancers, particularly of the digestive system, are preventable, so the current review focuses on the chemopreventative potential of the phytochemicals discussed. Ultimately, consumption of turmeric and other foods rich in phytochemicals should be encouraged to reduce the incidence of cancer.
Keywords: Cancer, colorectal cancer, chemoprevention, turmeric, curcumin, phytochemical

Colorectal cancer (CRC) is the third most common cancer and second leading cause of cancer-related deaths worldwide (WHO, 2022). Substantial global variation exists in the incidence of CRC; in a recent systematic analysis for the Global Burden of Disease Study (GBD), it was reported that Australasia, high-income Asia Pacific and high-income North America had the highest age-standardised incidence rates of CRC in 2017, while south Asia, western Sub-Saharan Africa and central Sub-Saharan Africa had the lowest (GBD, 2017). At least part of this discrepancy is believed to be attributable to Westernised lifestyles – including poor diet and inadequate physical activity, which are associated with risk factors for CRC (WCRF, 2018). As such, there is a significant body of research investigating CRC risk factors, and subsequently, recommendations to avoid and reduce these exposures have been given by organisations such as the Centers for Disease Control and Prevention (2022) and the World Cancer Research Fund (WCRF, 2018).
Less attention has been paid to investigating protective factors – that is, those whose exposure has a chemopreventative effect. However, the growing body of research in this area has produced some promising findings. Turmeric, the dried rhizome of the herbaceous perennial plant Curcuma longa Linn, is the focus of the current review. Found mostly in Asia, it has been used for centuries for culinary and traditional uses (Gupta et al., 2013). However, more recently, it has entered the realms of scientific research and its biological activities have been investigated.
Since curcumin was identified as the major bioactive compound in the spice (Kuttan et al., 1985), its multi-targeted chemopreventative activities have been thoroughly investigated. Subsequently, other bioactive compounds present in turmeric have also been identified and studied in this context. However, despite its potent chemopreventative potential, curcumin has not yet been approved for therapeutic use, primarily due to poor bioavailability. Various delivery system developments have been tested to tackle these limitations. One such method is the use of adjuvants, and numerous studies have found that concomitant administration of other phytochemicals with curcumin can improve its bioavailability. Given that turmeric contains many phytochemicals, this review proposes that more research should be done to uncover the interactions between them. Besides, there is growing evidence that turmeric may be a superior chemopreventative agent than curcumin alone.
The chemopreventative activities of curcumin (Giordano and Tommonaro, 2019) and of non-curcuminoids (Nair et al., 2019) have been reviewed elsewhere, as have the reasons for and methods to improve the bioavailability of curcumin (Liu et al., 2020; Sabet et al., 2021). While this review will provide an overview of these areas and discuss some relevant aspects, the reader is pointed towards these reviews for in-depth discussion of these topics. The aim of this article is to bring together areas of research relevant to the chemopreventative potential of turmeric. To the best of my knowledge, and at the time of writing, this is the first review to combine these topics. The paper begins by describing the bioactive compounds in turmeric and their individual chemopreventative activities, followed by evidence of synergism between them and methods to improve bioavailability. Finally, future directions for research into the potential of turmeric for cancer prevention and treatment are proposed.
It has been reported that more than two-thirds of human cancers could be prevented by lifestyle changes, and that dietary factors alone contribute to approximately 35 per cent of human cancer mortality (T. Y. Lee and Tseng, 2020a). For CRC specifically, it is reported that 90 per cent of deaths could be prevented with dietary intervention (Bolat et al., 2020). Despite this, chemoprevention is not often the focus of research efforts.
Many chemopreventative agents are anti-mutagens. Anti-mutagenicity may be exhibited through direct interaction with carcinogens to prevent them from binding DNA or by interfering with enzyme systems to prevent their metabolism. Alternatively, many chemopreventative agents exhibit their anti-cancer activities by acting on signalling pathways. Studies have shown that turmeric exhibits anti-mutagenicity (Polasa et al., 1992; Shalini and Srinivas, 1987, 1990; Srinivas and Shalini, 1991) and interacts with a variety of signalling molecules (Kim et al., 2012; Li et al., 2018, 2021).
Due to issues of drug resistance, toxicity and severe side effects from standard chemotherapy, there is growing interest in natural, non-toxic and safe compounds that possess anti-cancer potential (G.-Y. Lee et al., 2020). Phytochemicals are natural, biologically active compounds derived from plants, and extensive research has begun in this area of medicine. Phytochemicals that have been investigated for medical purposes are sometimes called ‘nutraceuticals’, a combination of the words ‘nutrition’ and ‘pharmaceutical’. Many phytochemicals have been shown to possess anti-cancer activities through various mechanisms, including regulating epigenetic changes, inhibiting metastasis, inhibiting cell cycle progression, inhibiting cell signal transduction and promoting apoptosis (T. Y. Lee and Tseng, 2020). While most studies have focused on the potential use of phytochemicals as chemosensitisers and adjuvants in therapy, there is also evidence of their chemopreventative abilities. Turmeric contains many phytochemicals, and it is these that are the focus of this review.
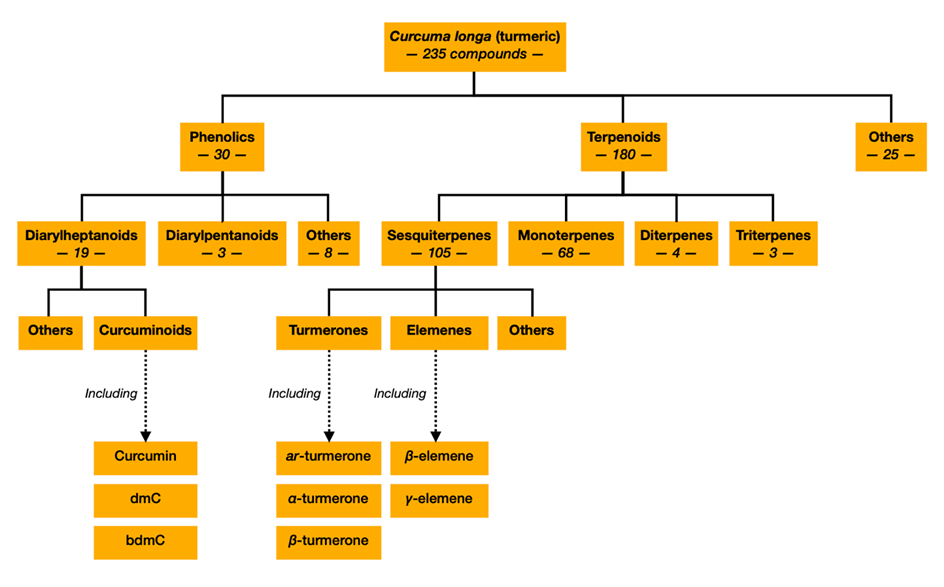
There have been 235 compounds identified in turmeric, most of which are either phenolic compounds or terpenoids (Figure 2). Phenolic compounds include diarylheptanoids, diarylpentanoids and some others, while terpenoids include monoterpenes, sesquiterpenes, diterpenes and triterpenes (Li, 2011). Curcuminoids are a type of diarylheptanoids, and the term ‘non-curcuminoids’ is used to encompass all other components of turmeric. It is now well-established that curcuminoids and some non-curcuminoids exhibit anti-cancer activities. This will be discussed later.
In 1985, the first study demonstrating the anti-cancer potential of turmeric was published. Ethanolic turmeric extract (ETE) was found to be highly cytotoxic to human leukaemic cells in vitro and also prevented animal tumours induced by Dalton’s lymphoma (Kuttan et al., 1985). The authors identified curcumin as being the cytotoxic component and suggested that it may be responsible for the anti-cancer effects of turmeric. In 1987, a follow-up study was published where topical application of ETE to cancerous lesions of human participants had beneficial effects in most patients (Kuttan et al., 1987). This was the first time that turmeric had been studied on human cancer patients. Since these initial findings, many studies have been conducted investigating the anti-cancer potential of turmeric. Given that curcumin had already been identified as the major active ingredient, it became the focus of much of the research that has been done over the past few decades.
Curcuminoids are the polyphenolic phytochemicals responsible for the yellow-orange colour of turmeric. The total curcuminoid content of turmeric rhizomes is thought to vary from about 2.8 to 10.9 per cent, but this drops to 0.58 to 3.15 per cent in commercial turmeric powders (Li, 2011). The term ‘curcumin’ is often used interchangeably with ‘curcuminoids’; however, curcumin is not the only curcuminoid present in turmeric. Curcumin (diferuloylmethane) has been reported to constitute about 77 per cent of the curcuminoid content of turmeric, while demethoxycurcumin (dmC) and bisdemethoxycurcumin (bdmC) constitute 17 per cent and 6 per cent, respectively (Anand et al., 2007). However, these figures vary slightly across the literature, partly due to variation among different C. longa species. Isolation of pure curcumin from turmeric is difficult and time-consuming, so commercial curcumin is usually a mix of these three curcuminoids (Li, 2011). Many studies investigating the effects of curcumin also use this mixture, although some do isolate the different curcuminoids.
Curcumin is by far the most studied curcuminoid. It was first isolated from turmeric in 1815 (Vogel and Pelletier, 1815) and has since been extensively studied for its therapeutic potential. Its purported biological activities include anti-inflammatory, anti-ulcer, anti-viral, anti-bacterial, anti-protozoal, anti-venom, antioxidant, anti-coagulant, anti-hypertensive, anti-hypocholesteraemic, anti-fibrotic, anti-mutagenic, anti-infertility and anti-cancer (Nair et al., 2019). The anti-cancer activities of curcumin are multi-targeted, interacting directly with some proteins and modulating the expression of others (Kunnumakkara et al., 2008). Studies have demonstrated its role in modulating transcription factors (e.g. NF-κB), growth factors (e.g. VEGF), enzymes (e.g. COX-2), kinases (e.g. cyclin D1) and inflammatory cytokines (e.g. TNF), as well as upregulation of pro-apoptotic proteins (e.g. Bax) and downregulation of anti-apoptotic proteins (e.g. Bcl-2) (Shehzad et al., 2013).[1] Due to the large quantity of research into the chemopreventative activities of curcumin, there are a significant number of reviews on the topic (Oyagbemi et al., 2009; Reuter et al., 2008; Thangapazham et al., 2006; Vallianou et al., 2015).
Despite the fact that curcumin had been identified as the major active ingredient of turmeric, and studies had demonstrated its potent anti-cancer potential, a few studies in the 1990s investigated the activities of curcumin-free turmeric. Early findings indicated that curcumin is the significant chemopreventative agent in turmeric. For example, in a study that assessed the inhibitory effects of aqueous turmeric extract (ATE) and curcumin-free aqueous turmeric extract (CFATE) on the mutagenicity of direct- and indirect-acting mutagens, only ATE was effective (Azuine et al., 1992). In another study, cytochrome P450 enzyme activity as well as DNA-adduct formation was found to be reduced only by curcumin-containing treatments and not by curcumin-free treatments (Deshpande and Maru, 1995). However, some studies found that curcumin-free treatments appeared more effective than curcumin-containing treatments. A study investigating the inhibitory effects of ATE, CFATE and curcumin on micronuclei formation in mice found CFATE to be the most effective (Azuine et al., 1992). Further, in a B[a]P-induced forestomach tumour model in mice,[2] CFATE reduced the tumour multiplicity and incidence to a greater extent than turmeric or ETE (Deshpande et al., 1997). However, when a similar experiment was conducted on a DMBA-induced mammary tumour mode in rats,[3] CFATE was found to be ineffective while ETE and turmeric significantly reduced tumour multiplicity, burden and incidence (Deshpande et al., 1998). While these findings are conflicting, they provided some indication that compounds other than curcumin may contribute to the chemopreventative activities of turmeric. This led to research into the other bioactive compounds in the spice.
Non-curcuminoids include all the bioactive compounds in turmeric except curcuminoids. These include phenolic compounds, terpenoids and some others. Non-curcuminoid phenolic compounds include some diarylheptanoids, diarylpentanoids and phenylpropenes, while terpenoids include monoterpenes, sesquiterpenes, diterpenes and triterpenes (Figure 2). Many of these have now been studied for their pharmacological activities and have been found to exhibit chemopreventative activities. These have been extensively reviewed elsewhere (Nair et al., 2019).
There is evidence that curcuminoids and sesquiterpenes show synergistic effects (Nishiyama et al., 2005; Yue et al., 2016), and this will be discussed later. There have been 109 sesquiterpenes identified in turmeric, and they are primarily found in turmeric oil (Li, 2011). Perhaps the most studied sesquiterpenes are turmerones and elemenes, of which ar-turmerone and β-elemene appear the most promising isoforms. Several studies have demonstrated the chemopreventative activities and therapeutic potential of these phytochemicals (Table 1). However, acknowledgement of this is only very recent and not yet widespread.
| Sesquiterpene | Studies showing chemopreventative potential | Reference |
| ar-turmerone | Promoted apoptosis in human chronic myelogenous leukaemia, rat basophilic leukaemia, human histiocytic lymphoma and mouse lymphocytic leukaemia. | (Ji et al., 2004) |
| Upregulated MMP-9 and COX-2 by blocking NF-κB, PI3K/Akt and ERK1/2 signalling and inhibited invasion, migration and colony formation in human breast cancer cells. | (Park et al., 2012) | |
| Induced apoptosis via ROS generation-mediated activation of ERK and JNK kinases in hepatocellular carcinoma cells. | (Cheng et al., 2012) | |
| Had cytotoxic effects on lymphocytic leukaemia and myeloid cells. | (Kim et al., 2013) | |
| β-elemene | Induced apoptosis and protective autophagy by inhibiting PI3K/Akt/m-TOR/p70S6K1 signalling in non-small cell lung cancer cells. | (Liu et al., 2012) |
| Inhibited proliferation and induced apoptosis and autophagy by inhibiting MAPK/ERK and PI3K/Akt/m-TOR signalling in renal cell carcinoma cells. | (Zhan et al., 2012) | |
| Reduced proliferation, promoted apoptosis and impaired invasiveness in glioblastoma cells. | (Xu et al., 2006) |
Despite its well-established potent bioactivity, tolerance and safety at high doses, curcumin has not been approved for use as a therapeutic agent due to its poor oral bioavailability (Anand et al., 2007; Liu et al., 2020; Sabet et al., 2021). While there are other possible routes of delivery (intravenous, nasal, topical, subcutaneous, intraperitoneal), oral administration is the preferred method of drug delivery because of its convenience, high patient compliance, cost-effectiveness and ease of production (Liu et al., 2020). Reasons for the poor oral bioavailability of curcumin and recent developments in delivery systems to tackle them are discussed below.
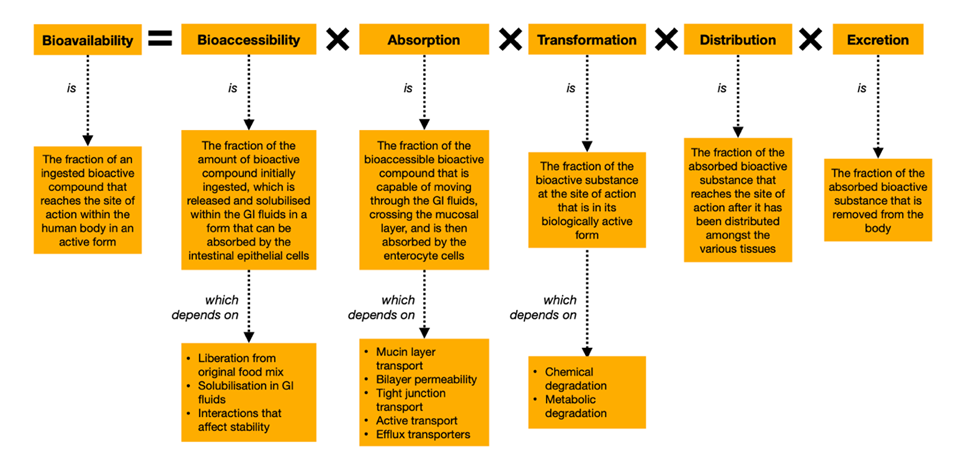
According to the Nutraceutical Bioavailability Classification Scheme (NuBACS), the bioavailability of a bioactive compound is dependent on five classes of physiochemical and physiological factors, namely bioaccessibility, absorption, transformation, distribution and excretion (Figure 3). The factors thought to be the main contributors to curcumin’s poor bioavailability are its low bioaccessibility, due to poor liberation from plant cell tissues as well as poor solubility in gastrointestinal (GI) fluids, and its transformation into less bioactive products through chemical and metabolic degradation (Sabet et al., 2021). These are discussed further below.
Bioaccessibility: For any orally administered drug to be bioaccessible, it needs to dissolve in GI fluids. GI fluids are acidic, but the aqueous solubility of curcumin is very low in acidic pH (Liu et al., 2020).
Transformation: Curcumin undergoes two phases of metabolic degradation. In Phase I, curcumin is reduced to the metabolites dihydrocurcumin, tetrahydrocurcumin and hexahydrocurcumin by reductases in the liver (Hoehle et al., 2007; C Ireson et al., 2001) and potentially by E. coli in the small intestine and colon (Hassaninasab et al., 2011). In Phase II, curcumin and its metabolites undergo glucuronidation and sulfation, which are catalysed by UDP-glucuronosyltransferase (UGT) and sulfotransferase (SULT), respectively (Ireson et al., 2002), producing curcumin sulfate, curcumin glucuronide sulfate, curcumin glucuronide, dihydro curcumin glucuronide, tetrahydro curcumin glucuronide and hexahydro curcumin glucuronide (Liu et al., 2020). The metabolites of curcumin are thought to be biologically active but substantially less so than curcumin (Zhu et al., 2017).
As discussed above, improving bioaccessibility and reducing transformation should be the focus of delivery system developments for curcumin. Recent studies have trialled a range of delivery methods that have now been the subject of multiple reviews (Araiza-Calahorra et al., 2018; Jiang et al., 2020; Liu et al., 2020; McClements, 2018, 2020). These methods include nanoparticles, liposomes, micelles, emulsions, solid dispersions and adjuvants (Liu et al., 2020). As this review focuses on phytochemicals, the use of them as adjuvants for curcumin is further discussed below.
In pharmacology, an adjuvant is a drug that is co-administered with another drug to enhance its efficacy. Many phytochemicals, such as those in Table 2, have been shown to exhibit chemopreventative activities, and these have been reviewed elsewhere (lycopene: Rowles et al., 2017; piperine: Zadorozhna, Tataranni and Mangieri, 2019; quercetin: Tang et al., 2020; resveratrol: Jiang et al., 2017; silibinin: Jahanafrooz et al., 2018; sulforaphane: Nandini et al., 2020). One recent study assessed the individual and combined effects of quercetin, curcumin, lycopene and sulforaphane on the viability and proliferation of normal and cancerous colon cells. The authors stated that the similar mechanisms by which these four phytochemicals exhibit their anti-cancer activities ‘encourages reflection on their impact on the human body when used jointly’ (Langner et al., 2019). Interestingly, they found that the anti-proliferative effect of the combination mix was greater than the sum of the individual effects of each of the four phytochemicals in HT-29 cells. While the specific interactions of these four compounds with each other were not investigated, the results indicated that there was some synergism among them. The authors proposed this may be because each compound used exhibits anti-cancer activities via different molecular mechanisms, but it should be noted that it is possible that one or more compounds were acting as adjuvants.
Several phytochemicals have been studied specifically as potential adjuvants for curcumin. Piperine is perhaps the most extensively studied and has been shown to improve the bioavailability of curcumin in numerous studies (Table 3). Piperine, quercetin and silibinin have been shown to inhibit UGTs in the liver (Grancharov et al., 2001; Reen et al., 1993; Williams et al., 2002), and piperine is also thought to inhibit SULTs (Zeng et al., 2017). A recent study found that silibinin, quercetin and tangeretin all reduced curcumin glucuronidation in mouse liver microsomes but, contrary to other studies, piperine did not (Grill et al., 2014). In the same study, an in vivo assessment using mice showed that quercetin, silibinin and piperine were effective at improving post-administration plasma levels of curcumin. The authors proposed that the improved bioavailability of curcumin by piperine in mice may be a result of its effect on curcumin absorption rather than its ability to inhibit its metabolism. Previous evidence has demonstrated that piperine can inhibit P-glycoprotein, an efflux transporter in intestinal epithelial cells that reduces the absorption of curcumin (Bhardwaj et al., 2002). Another study showed that piperine could affect the membrane dynamics and permeation characteristics of the intestines, which could improve its permeability and lead to increased absorption of drugs (Khajuria et al., 2002). However, more research is needed to establish the underlying mechanisms that are responsible for the observed improvements in bioavailability.
The relatively large fraction of orally administered curcumin that is not absorbed by the intestinal epithelial cells ends up passing through the colon and rectum to be egested in faeces. Thus, curcumin may be of most use for CRC. Many studies have focused their investigations on this area of the digestive system and used CRC cell lines and xenografts to study the chemopreventative activities of curcumin. These have been extensively reviewed (Johnson and Mukhtar, 2007; Weng and Goel, 2020). However, by the time curcumin reaches the colorectal area, it has been subjected to a significant amount of metabolic degradation. Thus, it would still be beneficial to reduce the transformation of curcumin through delivery systems like the ones described previously. The use of nanoformulations of curcumin specifically for the treatment of CRC has been recently reviewed (Wong et al., 2019).
| Name | Type | Sources |
| Piperine | Polyphenol | Long pepper and black pepper |
| Quercetin | Polyphenol | Various fruits and vegetables Examples: apples, onions |
| Silibinin | Polyphenol | Milk thistle |
| Resveratrol | Polyphenol | Various fruits and vegetables Examples: red grapes, red onion |
| Lycopene | Carotenoid | Various fruits and vegetables Examples: tomatoes, watermelon |
| Sulforaphane | Carotenoid | Cruciferous vegetables Examples: broccoli, cabbage |
| Studies using piperine as an adjuvant for curcumin | Reference |
| Piperine increased the serum concentration and time to maximum and decreased the elimination half-life and clearance of curcumin in rats and humans. | (Shoba et al., 1998) |
| Piperine enhanced the effect of curcumin on breast stem cell self-renewal. | (Kakarala et al., 2010) |
| Piperine attenuated the morphological, histopathological, biochemical, apoptotic and proliferative changes in the liver and serum by curcumin. | (Patial et al., 2015) |
| Piperine potentiated the inhibition of mTORC1* signalling by curcumin in human intestinal epithelial cells. | (Kaur et al., 2018) |
| PiperineEmulsomes additively contributed to the chemopreventative effects of CurcaEmulsomes on HCT116 cells. | (Bolat et al., 2020) |
As discussed previously, there is strong evidence that co-delivery of phytochemicals can improve the bioavailability and therefore the chemopreventative activities of curcumin. Given that turmeric naturally contains many phytochemicals (Figure 2), it is reasonable to hypothesise that treatment of turmeric in its entirety may be a more effective chemopreventative agent than just curcumin. A handful of studies have investigated this line of reasoning, and these are discussed below.
Carotenoids are a group of phytochemicals that belong to the tetraterpene family and are known to exhibit anti-cancer activities (Milani et al., 2017). Previously, the carotenoids lycopene and sulforaphane were mentioned regarding a study where a mix of lycopene, sulforaphane, quercetin and curcumin showed synergistic effects on colon cancer cells (Langner et al., 2019). While the different phytochemicals were not tested for their individual synergistic effects with curcumin, two out of three of the phytochemicals delivered with curcumin were terpenes. To that end, it is reasonable to propose that other terpenes may also show synergistic effects with curcumin. Turmeric does not contain tetraterpenes, but does contain a large array of other types of terpenes, such as sesquiterpenes (Figure 2). In fact, sesquiterpenes and curcuminoids in turmeric have been indicated to have synergistic effects in relation to blood glucose levels in type II diabetes (Nishiyama et al., 2005). While there has not been much research into the interactions of the phytochemicals in turmeric, a handful of studies have compared the bioavailability and the chemopreventative potential of curcumin administered alone or in turmeric (with an equal amount of curcumin).
In one study, the effects of curcumin and ETE were compared in colon-cancer xenograft-bearing mice. While curcumin inhibited tumour growth by 26.6 per cent, ETE inhibited it by 38.9 per cent (Yue et al., 2016). ETE also significantly reduced tumour weight and increased the apoptotic area to a greater extent than curcumin, but quantitative results were not reported. In the same study, the presence of turmerones was shown to enhance the inhibitory effects of curcumin on human colon cancer cell lines, and it was concluded that there was a strong indication of synergism. In another study, turmeric was found to be more potent than curcumin at inhibiting the growth of various human cancer cell lines, namely myelogenous leukaemia, colon adenocarcinoma, pancreatic cancer, breast and multiple myeloma (Kim et al., 2012). Interestingly, in a study that separated the three major curcuminoids in turmeric (curcumin, dmC and bdmC), turmeric extract exhibited a similar level of inhibition of human lung cancer cell viability to dmC and bdmC at lower concentrations (2–5µg/ml) but was more inhibitory at higher concentrations (Kukula-Koch et al., 2018). At 10µg/ml, turmeric extract inhibited cell viability by 70 per cent, while the same concentration of dmC and bdmC showed 60 per cent inhibition, and curcumin showed hardly any. This was consistent with other studies which had investigated individual curcuminoids and found dmC and bdmC to be more effective chemopreventative agents than curcumin (Basile et al., 2009; Thapliyal and Maru, 2001; Yodkeeree et al., 2009).
These studies demonstrated that turmeric exhibited more potent chemopreventative activities than curcumin alone, but whether this was a result of the cumulative anti-cancer activities of individual compounds or because of adjuvant/synergistic effects was not able to be established. However, some studies have investigated the bioavailability of curcumin when administered alone or in turmeric (with an equal amount of curcumin). One study found that a turmeric diet given to rats led to a significantly higher concentration of curcumin in the intestines than a curcumin diet (Martin et al., 2012). This led the authors to conclude that curcumin delivered in turmeric had a higher bioavailability than curcumin alone. While the authors did not specify, it appears that the measurements were of curcumin only and not its metabolites. Thus, the markedly higher concentration of curcumin in the intestine when it was administered in turmeric suggests either that it was more bioaccessible (perhaps more soluble in GI fluids) and/or that it was more protected from metabolic degradation. Additionally, the smaller yet notable increase in curcumin concentration in serum indicates that more curcumin was absorbed when it was administered in turmeric. This is consistent with findings by another set of researchers who investigated the role of turmerones, a sub-group of sesquiterpenes, in turmeric. In one study, an in vitro Caco-2 cell monolayer model was used to investigate the effect of turmerones on the transport of curcumin, and it was found that the presence of turmerones increased the accumulation of curcumin inside colonic cells (Yue et al., 2012). Another study investigated the effects of turmeric and curcumin on glucuronidation (catalysed by UGTs) and sulfation (catalysed by SULTs) in Caco-2 cells. Given that these conjugation reactions are involved in both the metabolic activation of carcinogens and the metabolic degradation of curcumin, a higher level of inhibition is desirable. They found that turmeric and curcumin inhibited sulfation to a similar extent, but that turmeric inhibited glucuronidation more so than curcumin (Naganuma et al., 2006). This indicates that compounds in turmeric other than curcumin may also inhibit glucuronidation and could be one of the mechanisms underlying the improved bioavailability of curcumin when it is delivered in turmeric.
Together, these studies provide evidence that other compounds in turmeric can improve the bioavailability of curcumin; turmerones can improve its absorption in the intestines and other compounds could be involved in inhibiting its metabolic degradation, but it is not yet known which. The improved bioavailability of curcumin in turmeric could at least partly account for the improved chemopreventative potential of turmeric that has been demonstrated; however, it is also possible that the cumulative anti-cancer activities of the compounds in turmeric contribute to this superiority. More research should be done to address these uncertainties and to deduce the underlying mechanisms.
The chemopreventative effects of curcumin are well-established in the literature, but its low bioavailability has presented issues of clinical efficacy. It has been demonstrated by several studies that treatment with turmeric can lead to better therapeutic outcomes than treatment with curcumin alone. Given the chemopreventative effects of many non-curcuminoids, it is likely that there is a cumulative effect of the chemopreventative activities of the compounds in turmeric. However, a handful of studies have shown that curcumin delivered in turmeric may have improved oral bioavailability than when it is administered alone, and so this is likely to contribute to the improved chemopreventative effects of turmeric shown in other studies. The potential for phytochemicals to act as adjuvants to improve the bioavailability of curcumin has been well-established. However, given the small number of studies comparing the chemopreventative potential and bioavailability of turmeric and curcumin, and the even smaller number investigating the interactions underlying this, more investigation should be done to confirm these findings to establish whether turmeric truly is the superior treatment. If this proves to be the case, it should be the focus of clinical trials rather than curcumin alone. This view is shared by the authors of some of the key studies discussed previously, and was expressed in their recent review (Lau and Yue, 2020).
The use of phytochemicals is an area of interest for both cancer prevention and treatment. There is a need for alternative and adjuvant therapeutics due to the severe side effects of traditional treatments as well as the emerging and serious issue of chemotherapeutic drug resistance. However, as mentioned previously, it is reported that a significant proportion of cancers can be prevented through diet, particularly those cancers of the digestive system such as colorectal cancer. Thus, more focus should be aimed at chemoprevention. Given their natural presence in the diet, increased intake of turmeric and other foods containing phytochemicals should be encouraged to reduce the incidence of cancer.
Thank you to my supervisor, Dr Gillian Campling, for your much appreciated support and guidance throughout the process of writing this review.
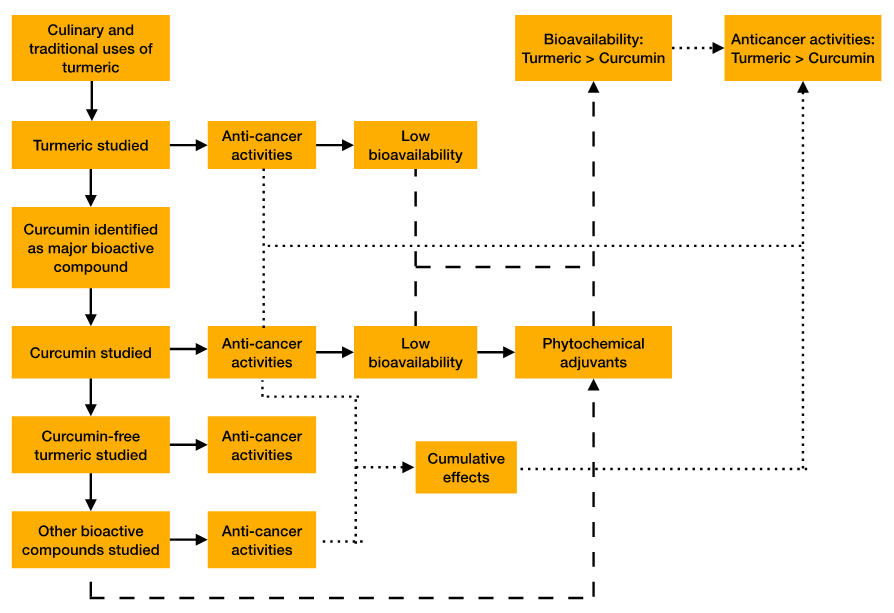
Figure 1: Graphical abstract illustrating the scope of the review
Figure 2: Compounds identified in Curcuma longa (turmeric). Figure constructed by author, using information from (Li, 2011)
Figure 3: The five classes of factors that contribute to the overall bioavailability of an orally administered bioactive compound, according to NuBACS (McClements, 2018). Figure constructed by author, using information from Sabet et al. (2021)
Table 1: Examples of studies demonstrating chemopreventative activities of ar-turmerone and β-elemene
Table 2: Examples of phytochemicals that have chemopreventative activities and have been trialled as adjuvants for curcumin
Table 3: Examples of studies testing piperine as an adjuvant for curcumin
[1] NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; VEGF, vascular endothelial growth factor; COX, cyclooxygenase; TNF, tumour necrosis factor
[2] B[a]P, benzo[a]pyrene
[3] DMBA, 7,12-dimethylbanz(a)anthracene
Anand, P., A. B. Kunnumakkara, R. A. Newman and B. B. Aggarwal (2007), 'Bioavailability of curcumin: Problems and promises', Molecular Pharmaceutics, 4 (6), 807–18
Araiza-Calahorra, A., M. Akhtar and A. Sarkar (2018), 'Recent advances in emulsion-based delivery approaches for curcumin: From encapsulation to bioaccessibility', Trends in Food Science & Technology, 71, 155–69.
Azuine, M. A., J. J. Kayal and S. V. Bhide (1992), 'Protective role of aqueous turmeric extract against mutagenicity of direct-acting carcinogens as well as benzo [alpha] pyrene-induced genotoxicity and carcinogenicity', Journal of Cancer Research and Clinical Oncology, 118 (6), 447–52
Basile, V., E. Ferrari, S. Lazzari, S. Belluti, F. Pignedoli and C. Imbriano (2009), 'Curcumin derivatives: Molecular basis of their anti-cancer activity', Biochemical Pharmacology, 78 (10), 1305–15
Bhardwaj, R. K., H. Glaeser, L. Becquemont, U. Klotz, S. K. Gupta and M. F. Fromm, (2002), 'Piperine, a major constituent of black pepper, inhibits human P-glycoprotein and CYP3A4', The Journal of Pharmacology and Experimental Therapeutics, 302 (2), 645–50
Bolat, Z. B., Z. Islek, B. N. Demir, E. N. Yilmaz, F. Sahin and M. H. Ucisik (2020), 'Curcumin- and Piperine-Loaded Emulsomes as Combinational Treatment Approach Enhance the Anticancer Activity of Curcumin on HCT116 Colorectal Cancer Model', Frontiers in Bioengineering and Biotechnology, 8, 50
Centers for Disease Control and Prevention (2022), ‘Risk factors & symptoms; colorectal cancer screening saves lives’, available at: https://www.cdc.gov/cancer/colorectal/, accessed 14 October 2022
Cheng, S. B., L. C. Wu, Y. C. Hsieh, C. H. Wu, Y. J. Chan, L. H. Chang, C. M. J. Chang, S. L. Hsu, C. L. Teng and C. C. Wu (2012), 'Supercritical carbon dioxide extraction of aromatic turmerone from Curcuma longa Linn. induces apoptosis through reactive oxygen species-triggered intrinsic and extrinsic pathways in human hepatocellular carcinoma HepG2 cells', Journal of Agricultural and Food Chemistry, 60 (38), 9620–30
Deshpande, S. S., A. D. Ingle and G. B. Maru (1997), 'Inhibitory effects of curcumin-free aqueous turmeric extract on benzo[a]pyrene-induced forestomach papillomas in mice', Cancer Letters, 118 (1), 79–85
Deshpande, S. S., A. D. Ingle and G. B. Maru (1998), 'Chemopreventive efficacy of curcumin-free aqueous turmeric extract in 7,12-dimethylbenz[a]anthracene-induced rat mammary tumorigenesis', Cancer Letters, 123 (1), 35–40
Deshpande, S. S. and G. B. Maru (1995), 'Effects of curcumin on the formation of benzo[a]pyrene derived DNA adducts in vitro', Cancer Letters, 96 (1), 71–80
GBD (Global Burden of Disease Study) (2017), ‘The global, regional, and national burden of colorectal cancer and its attributable risk factors in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017’, The Lancet Gastroenterology & Hepatology, 4 (12), 913–33
Giordano, A. and G. Tommonaro (2019), 'Curcumin and Cancer', Nutrients, 11 (10)
Grancharov, K., Z. Naydenova, S. Lozeva and E. Golovinsky (2001), 'Natural and synthetic inhibitors of UDP-glucuronosyltransferase', Pharmacology & Therapeutics, 89 (2), 171–86
Grill, A. E., B. Koniar and J. Panyam (2014), 'Co-delivery of natural metabolic inhibitors in a self-microemulsifying drug delivery system for improved oral bioavailability of curcumin', Drug Delivery and Translational Research, 4 (4), 344–52
Gupta, S. C., B. Sung, J. H. Kim, S. Prasad, S. Li and B. B. Aggarwal (2013), 'Multitargeting by turmeric, the golden spice: From kitchen to clinic', Molecular Nutrition and Food Research, 57 (9), 1510–28
Hassaninasab, A., Y. Hashimoto, K. Tomita-Yokotani and M. Kobayashi (2011), 'Discovery of the curcumin metabolic pathway involving a unique enzyme in an intestinal microorganism', Proceedings of the National Academy of Sciences, 108 (16), 6615 LP – 6620
Hoehle, S. I., E. Pfeiffer and M. Metzler (2007), 'Glucuronidation of curcuminoids by human microsomal and recombinant UDP-glucuronosyltransferases', Molecular Nutrition & Food Research, 51 (8), 932–38
Ireson, C, S. Orr, D. J. Jones, R. Verschoyle, C. K. Lim, J. L. Luo, L. Howells, S. Plummer, R. Jukes, M. Williams, W. P. Steward and A. Gescher (2001), 'Characterization of metabolites of the chemopreventive agent curcumin in human and rat hepatocytes and in the rat in vivo, and evaluation of their ability to inhibit phorbol ester-induced prostaglandin E2 production', Cancer Research, 61 (3), 1058–64
Ireson, C., D. Jones, S. Orr, M. Coughtrie, D. Boocock, M. Williams, P. Farmer, W. Steward and A. Gescher (2002), 'Metabolism of the cancer chemopreventive agent curcumin in human and rat intestine', Cancer Epidemiology, Biomarkers & Prevention: A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology, 11, 105–11.
Jahanafrooz, Z., N. Motamed, B. Rinner, A. Mokhtarzadeh and B. Baradaran (2018), 'Silibinin to improve cancer therapeutic, as an apoptotic inducer, autophagy modulator, cell cycle inhibitor, and microRNAs regulator', Life Sciences, 213, 236–47
Ji, M., J. Choi, J. Lee and Y. Lee (2004), 'Induction of apoptosis by ar-turmerone on various cell lines', Int J Mol Med, 14 (2), 253–56
Jiang, T., W. Liao and C. Charcosset (2020), 'Recent advances in encapsulation of curcumin in nanoemulsions: A review of encapsulation technologies, bioaccessibility and applications', Food Research International, 132 (October 2019)
Jiang, Z., K. Chen, L. Cheng, B. Yan, W. Qian, J. Cao, J. Li, E. Wu, Q. Ma and W. Yang (2017), 'Resveratrol and cancer treatment: updates', Annals of the New York Academy of Sciences, 1403 (1), 59–69
Johnson, J. J. and H. Mukhtar (2007), 'Curcumin for chemoprevention of colon cancer', Cancer Letters, 255 (2), 170–81
Kakarala, M., D. E. Brenner, H. Korkaya, C. Cheng, K. Tazi, C. Ginestier, S. Liu, G. Dontu and M. S. Wicha (2010), 'Targeting breast stem cells with the cancer preventive compounds curcumin and piperine', Breast Cancer Research and Treatment, 122 (3), 777–85
Kaur, H., B. He, C. Zhang, E. Rodriguez, D. S. Hage and R. Moreau (2018), 'Piperine potentiates curcumin-mediated repression of mTORC1 signaling in human intestinal epithelial cells: implications for the inhibition of protein synthesis and TNFα signaling', The Journal of Nutritional Biochemistry, 57, 276–86
Khajuria, A., N. Thusu and U. Zutshi (2002), 'Piperine modulates permeability characteristics of intestine by inducing alterations in membrane dynamics: Influence on brush border membrane fluidity, ultrastructure and enzyme kinetics', Phytomedicine, 9 (3), 224–31
Kim, J. H., S. C. Gupta, B. Park, V. R. Yadav and B. B. Aggarwal (2012), 'Turmeric (Curcuma longa) inhibits inflammatory nuclear factor (NF)-κB and NF-κB-regulated gene products and induces death receptors leading to suppressed proliferation, induced chemosensitization, and suppressed osteoclastogenesis', Molecular Nutrition & Food Research, 56 (3), 454–65
Kim, D., Y. Suh, H. Lee and Y. Lee (2013), 'Immune activation and antitumor response of ar-turmerone on P388D1 lymphoblast cell implanted tumors', International Journal of Molecular Medicine, 31 (2), 386–92
Kukula-Koch, W., A. Grabarska, J. Łuszczki, L. Czernicka, E. Nowosadzka, E. Gumbarewicz, A. Jarząb, G. Audo, S. Upadhyay, K. Głowniak and A. Stepulak (2018), 'Superior anticancer activity is demonstrated by total extract of Curcuma longa L. as opposed to individual curcuminoids separated by centrifugal partition chromatography', Phytotherapy Research, 32 (5), 933–42
Kunnumakkara, A. B., P. Anand and B. B. Aggarwal (2008), 'Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins', Cancer Letters, 269 (2), 199–225
Kuttan, R., P. Bhanumathy, K. Nirmala and M. C. George (1985), 'Potential anticancer activity of turmeric (Curcuma longa)', Cancer Letters, 29 (2), 197–202
Kuttan, R., P. C. Sudheeran and C. D Josph (1987), 'Turmeric and curcumin as topical agents in cancer therapy', Tumori, 73 (1), 29–31.
Langner, E., M. K. Lemieszek W. Rzeski (2019), 'Lycopene, sulforaphane, quercetin, and curcumin applied together show improved antiproliferative potential in colon cancer cells in vitro', Journal of Food Biochemistry, 43 (4), e12802
Lau, C. B. S. and G. G. L. Yue, (2020), 'Adjuvant Value of Turmeric Extract (Containing Curcumin) in Colorectal Cancer Management BT - Natural Products for Cancer Chemoprevention: Single Compounds and Combinations', Springer International Publishing, 209–39
Lee, G. Y., J. S. Lee, C. G. Son and N. H. Lee, (2020), 'Combating Drug Resistance in Colorectal Cancer Using Herbal Medicines', Chinese Journal of Integrative Medicine
Lee, T. Y. and Y. H. Tseng (2020), 'The potential of phytochemicals in oral cancer prevention and therapy: A review of the evidence', Biomolecules, 10 (8), 1–30
Li, S. (2011), 'Chemical Composition and Product Quality Control of Turmeric (Curcuma longa L.)', Pharmaceutical Crops, 5 (1), 28–54
Li, M., G. G. L. Yue, S. K. W. Tsui, K. P. Fung and C. B. S. Lau (2018). 'Turmeric extract, with absorbable curcumin, has potent anti-metastatic effect in vitro and in vivo'. Phytomedicine: International Journal of Phytotherapy and Phytopharmacology, 46, 131–41
Li, M., G. G. L. Yue, L. Luo, S. K. W. Tsui, K. P. Fung, S. S. M. Ng and C. B. S. Lau, (2021), 'Turmeric Is Therapeutic in Vivo on Patient-Derived Colorectal Cancer Xenografts: Inhibition of Growth, Metastasis, and Tumor Recurrence', Frontiers in Oncology, 10 (January), 1–16
Liu, J., X. J. Hu, B. Jin, X. J. Qu, K. Z. Hou and Y. P. Liu (2012), 'β-Elemene induces apoptosis as well as protective autophagy in human non-small-cell lung cancer A549 cells', The Journal of Pharmacy and Pharmacology, 64 (1), 146–53
Liu, Z., J. D. Smart and A. S. Pannala (2020), 'Recent developments in formulation design for improving oral bioavailability of curcumin', Journal of Drug Delivery Science and Technology, 60 (September)
Martin, R. C. G., H. S. Aiyer, D. Malik and Y. Li (2012), 'Effect on pro-inflammatory and antioxidant genes and bioavailable distribution of whole turmeric vs curcumin: Similar root but different effects', Food and Chemical Toxicology, 50 (2), 227–31
McClements, D. J. (2018), 'Delivery by Design (DbD): A Standardized Approach to the Development of Efficacious Nanoparticle- and Microparticle-Based Delivery Systems', Comprehensive Reviews in Food Science and Food Safety, 17 (1), 200–19
McClements, D. J. (2020), 'Advances in nanoparticle and microparticle delivery systems for increasing the dispersibility, stability, and bioactivity of phytochemicals', Biotechnology Advances, 38, 107287
Milani, A., M. Basirnejad, S. Shahbazi and A. Bolhassani (2017), ‘Carotenoids: biochemistry, pharmacology and treatment', British Journal of Pharmacology, 174 (11), 1290–324
Naganuma, M., A. Saruwatari, S. Okamura and H. Tamura (2006), ‘'Turmeric and curcumin modulate the conjugation of 1-naphthol in Caco-2 cells', Biological & Pharmaceutical Bulletin, 29 (7), 1476–79
Nair, A., A. Amalraj, J. Jacob, A. B. Kunnumakkara and S. Gopi (2019), 'Non-curcuminoids from turmeric and their potential in cancer therapy and anticancer drug delivery formulations', Biomolecules, 9 (1)
Nandini, D. B., R. S. Rao, B. S. Deepak and P. B Reddy (2020), 'Sulforaphane in broccoli: The green chemoprevention!! Role in cancer prevention and therapy', Journal of Oral and Maxillofacial Pathology: JOMFP, 24 (2), 405
Nishiyama, T., T. Mae, H. Kishida, M. Tsukagawa, Y. Mimaki, M. Kuroda, Y. Sashida, K. Takahashi, T. Kawada, K. Nakagawa and M. Kitahara (2005), 'Curcuminoids and sesquiterpenoids in turmeric (Curcuma longa L.) Suppress an increase in blood glucose level in type 2 diabetic KK-Aγ mice', Journal of Agricultural and Food Chemistry, 53 (4), 959–63
Oyagbemi, A. A., A. B. Saba and A. O. Ibraheem (2009), 'MINI-REVIEW Curcumin: From Food Spice to Cancer Prevention', Cancer, 10, 963–68.
Park, S. Y., Y. H. Kim, Y. Kim and S. J. Lee (2012), 'Aromatic-turmerone attenuates invasion and expression of MMP-9 and COX-2 through inhibition of NF-κB activation in TPA-induced breast cancer cells', Journal of Cellular Biochemistry, 113 (12), 3653–62
Patial, V., M. S., S. Sharma, K. Pratap, D. Singh and Y. S. Padwad (2015). 'Synergistic effect of curcumin and piperine in suppression of DENA-induced hepatocellular carcinoma in rats', Environmental Toxicology and Pharmacology, 40 (2), 445–52
Polasa, K., T. C. Raghuram, T. P. Krishna and K. Krishnaswamy (1992), ‘Effect of turmeric on urinary mutagens in smokers’, Mutagenesis, 7 (2), 107–09
Reen, R. K., D. S. Jamwal, S. C. Taneja, J. L. Koul, R. K. Dubey, F. J. Wiebel and J. Singh (1993), ‘Impairment of UDP-glucose dehydrogenase and glucuronidation activities in liver and small intestine of rat and guinea pig in vitro by piperine’, Biochemical Pharmacology, 46 (2), 229–38
Reuter, S., S. Eifes, M. Dicato, B. B. Aggarwal and M. Diederich (2008), ‘Modulation of anti-apoptotic and survival pathways by curcumin as a strategy to induce apoptosis in cancer cells’, Biochemical Pharmacology, 76 (11), 1340–51
Rowles, J. L. 3rd, K. M. Ranard, J. W. Smith, R. An and J. W. J. Erdman (2017), ‘Increased dietary and circulating lycopene are associated with reduced prostate cancer risk: a systematic review and meta-analysis’, Prostate Cancer and Prostatic Diseases, 20 (4), 361–77
Sabet, S., A. Rashidinejad, L. D. Melton and D. J. McGillivray (2021), ‘Recent advances to improve curcumin oral bioavailability’, Trends in Food Science & Technology, 110, 253–66
Shalini, V. K. and L. Srinivas (1987), ‘Lipid peroxide induced DNA damage: protection by turmeric (Curcuma longa)’, Molecular and Cellular Biochemistry, 77 (1), 3–10
Shalini, V. K. and L. Srinivas (1990), ‘Fuel smoke condensate induced DNA damage in human lymphocytes and protection by turmeric (Curcuma longa)’, Molecular and Cellular Biochemistry, 95 (1), 21–30
Shehzad, A., J. Lee and Y. S. Lee (2013), ‘Curcumin in various cancers’, BioFactors, 39 (1), 56–68
Shoba, G., D. Joy, T. Joseph, M. Majeed, R. Rajendran P. S. Srinivas (1998), ‘Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers’, Planta Medica, 64 (4), 353–56
Srinivas, L. and V. K. Shalini (1991), ‘DNA damage by smoke: protection by turmeric and other inhibitors of ROS’, Free Radical Biology & Medicine, 11 (3), 277–83
Tang, S. M., X. T. Deng, J. Zhou, Q. P. Li, X. X. Ge and L. Miao (2020), ‘Pharmacological basis and new insights of quercetin action in respect to its anti-cancer effects’, Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie, 121, 109604
Thangapazham, R. L., A. Sharma and R. K. Maheshwari (2006), ‘Multiple molecular targets in cancer chemoprevention by curcumin’, AAPS Journal, 8 (3), 443–49
Thapliyal, R. and G. B. Maru (2001), ‘Inhibition of cytochrome P450 isozymes by curcumins in vitro and in vivo’, Food and Chemical Toxicology, 39 (6), 541–47
Vallianou, N. G., A. Evangelopoulos, N. Schizas and C. Kazazis (2015), ‘Potential anticancer properties and mechanisms of action of curcumin’, Anticancer Research, 35 (2), 645–51.
Vogel A. and J. Pelletier (1815), ‘Examen chimique de la racine de Curcuma’, Journal de Pharmacie
WCRF (World Cancer Research Fund/American Institute for Cancer Research) (2018), ‘Continuous Update Project Expert Report 2018. Diet, nutrition, physical activity and colorectal cancer’, available at https://www.dietandcancerreport.org, accessed 07 October 2022
WHO (World Health Organization) (2022), ‘Cancer Key Facts’, available at https://www.who.int/news-room/fact-sheets/detail/cancer, accessed 07 October 2022
Weng, W. and A. Goel (2020), ‘Curcumin and colorectal cancer: An update and current perspective on this natural medicine’, Seminars in Cancer Biology
Williams, J. A., B. J. Ring, V. E. Cantrell, K. Campanale, D. R. Jones, S. D. Hall and S. A. Wrighton (2002), ‘Differential Modulation of UDP-Glucuronosyltransferase 1A1 (UGT1A1)-Catalyzed Estradiol-3-glucuronidation by the Addition of UGT1A1 Substrates and Other Compounds to Human Liver Microsomes’, Drug Metabolism and Disposition, 30 (11), 1266–73
Wong, K. E., S. C. Ngai, K. G. Chan, L. H. Lee, B. H. Goh and L. H. Chuah (2019), ‘Curcumin Nanoformulations for Colorectal Cancer: A Review’, Frontiers in Pharmacology, 10, 152
Xu, L., S. Tao, X. Wang, Z. Yu, M. Wang, D. Chen, Y. Jing and J. Dong (2006), ‘The synthesis and anti-proliferative effects of beta-elemene derivatives with mTOR inhibition activity’, Bioorganic & Medicinal Chemistry, 14 (15), 5351–56
Yodkeeree, S., W. Chaiwangyen, S. Garbisa and P. Limtrakul (2009), ‘Curcumin, demethoxycurcumin and bisdemethoxycurcumin differentially inhibit cancer cell invasion through the down-regulation of MMPs and uPA’, The Journal of Nutritional Biochemistry, 20 (2), 87–95
Yue, G. G. L., S. W. Cheng, H. Yu, Z. S. Xu, J. K. M. Lee, P. M. Hon, M. Y. H. Lee, E. J. Kennelly, G. Deng, S. K. Yeung, B. R. Cassileth, K. P. Fung, P. C. Leung and C. B. S. Lau (2012), ‘The role of turmerones on curcumin transportation and P-glycoprotein activities in intestinal caco-2 cells’, Journal of Medicinal Food, 15 (3), 242–52
Yue, G. G. L., L. Jiang, H. F. Kwok, J. K. M. Lee, K. M. Chan, K. P. Fung, P. C. Leung and C. B. S Lau (2016), ‘Turmeric ethanolic extract possesses stronger inhibitory activities on colon tumour growth than curcumin - The importance of turmerones’, Journal of Functional Foods, 22, 565–77
Zadorozhna, M., T. Tataranni and D. Mangieri (2019), ‘Piperine: role in prevention and progression of cancer’, Molecular Biology Reports, 46 (5), 5617–29
Zeng, X., D. Cai, Q. Zeng, Z. Chen, G. Zhong, J. Zhuo, H. Gan, X. Huang, Z. Zhao, N. Yao, D. Huang, C. Zhang, D. Sun and Y. Chen (2017), ‘Selective reduction in the expression of UGTs and SULTs, a novel mechanism by which piperine enhances the bioavailability of curcumin in rat’, Biopharmaceutics & Drug Disposition, 38 (1), 3–19
Zhan, Y. H., J. Liu, X. J. Qu, K. Z. Hou, K. F. Wang, Y. P. Liu and B. Wu (2012), ‘β-Elemene induces apoptosis in human renal-cell carcinoma 786-0 cells through inhibition of MAPK/ERK and PI3K/Akt/ mTOR signalling pathways’, Asian Pacific Journal of Cancer Prevention: APJCP, 13 (6), 2739–44
Zhu, J., K. Z. Sanidad, E. Sukamtoh and G. Zhang (2017), ‘Potential roles of chemical degradation in the biological activities of curcumin’, Food & Function, 8 (3), 907–14
Adjuvant: A treatment that enhances an existing medical regimen, as a pharmacological agent added to a drug to increase or aid its effect (The American Heritage Medical Dictionary).
Apoptosis: Programmed cell death (Farlex and Partners Medical Dictionary).
Bioavailability: The degree to which a drug or other substance becomes available to the target tissue after administration (Miller-Keane Encyclopaedia and Dictionary of Medicine, Nursing, & Allied Health).
Carcinogen: A substance that causes cancer (Miller-Keane Encyclopaedia and Dictionary of Medicine, Nursing, & Allied Health).
Chemoprevention: The use of chemical agents, drugs, or food supplements to prevent the development of cancer (The American Heritage Medical Dictionary).
Chemosensitiser: Any of several compounds that make cells, especially tumour cells, sensitive to chemotherapeutic agents (The American Heritage Medical Dictionary).
Curcuminoid: Any of several polyphenols, including curcumin, found in turmeric and other species in the genus Curcuma (The American Heritage Medical Dictionary).
Cytotoxic: Of, relating to, or producing a toxic effect on cells (American Heritage Dictionary of the English Language).
Epigenetics: Changes in the way genes are expressed that occur without changes in the sequence of nucleic acids (Farlex and Partners Medical Dictionary).
Metastasis: A secondary cancerous growth formed by transmission of cancerous cells from a primary growth located elsewhere in the body (The American Heritage Medical Dictionary).
Mutagen: A substance or agent that can induce genetic mutation (Collins English Dictionary); Anti-mutagen A factor that interferes with the mutagenic actions or effects of a substance (Farlex Partner Medical Dictionary).
Phenolic: Of, relating to, containing, or derived from phenol, an aromatic organic compound (The American Heritage Medical Dictionary).
Phytochemical: A non-nutritive bioactive plant substance, such as a flavonoid or carotenoid, considered to have a beneficial effect on human health (also known as phytonutrient) (The American Heritage Medical Dictionary).
Proliferation: Growth and reproduction of similar cells (Medical Dictionary for the Health Professionals).
Synergistic: The combined action of two or more processes is greater than the sum of each acting separately (Gale Encyclopaedia of Medicine).
Terpenoid: A class of chemical compounds including all terpenes, a naturally occurring chemical compounds found in plants and some animals. (The American Heritage Medical Dictionary).
To cite this paper please use the following details: Bowers, M. (2022), 'Preventing Cancer with Turmeric: The Whole is Better than the Sum of its Parts', Reinvention: an International Journal of Undergraduate Research, Volume 15, Issue 2, https://reinventionjournal.org/article/view/939. Date accessed [insert date]. If you cite this article or use it in any teaching or other related activities please let us know by e-mailing us at Reinventionjournal@warwick.ac.uk.