Emily Hartley, University of Exeter
Harrison Moon, University of Exeter, University College Maastricht
Ana I. S. Neves, University of Exeter
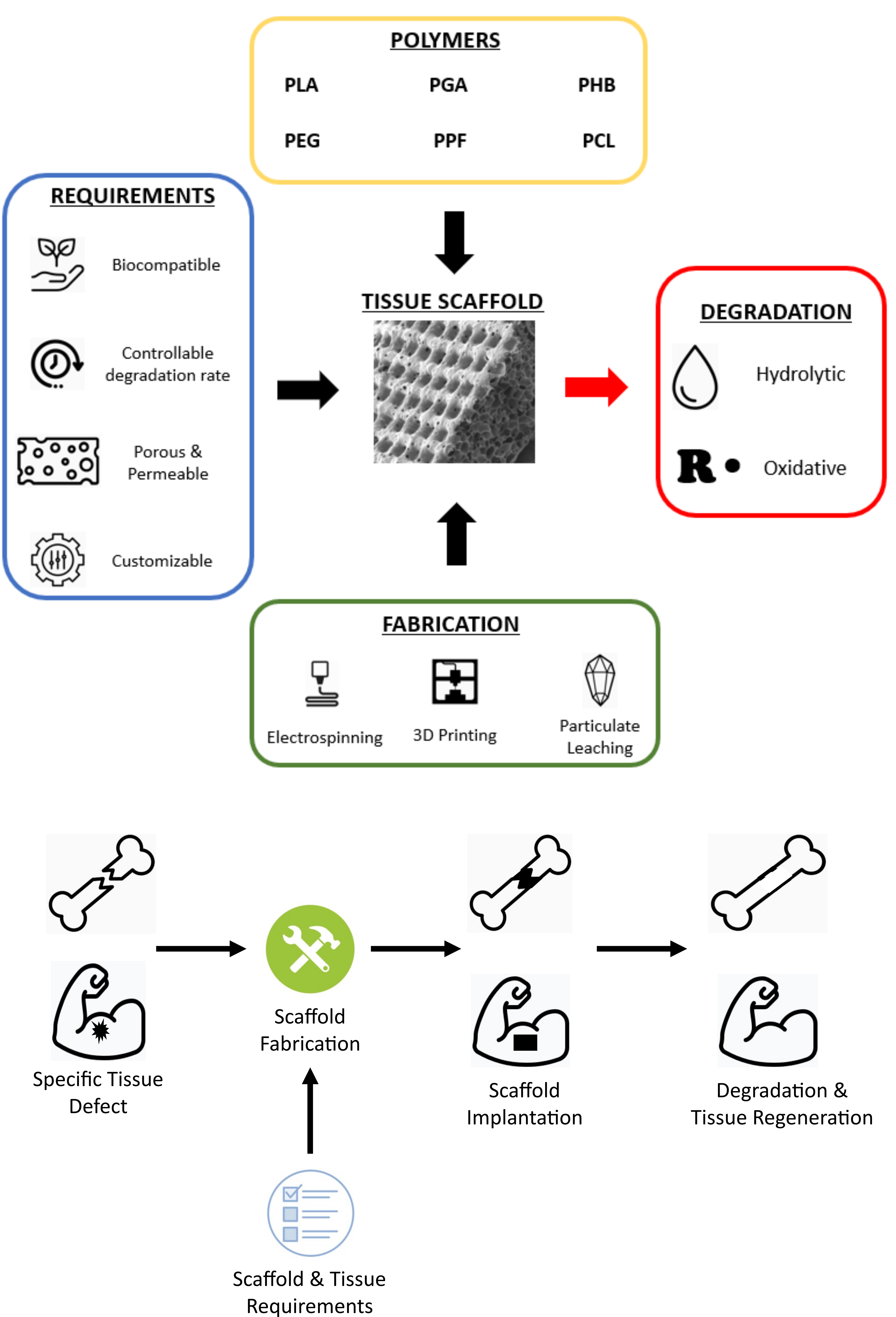
Tissue engineering is a revolutionary area of medicine, helping the body to heal large quantities of tissue loss that would otherwise require grafting procedures to promote recovery. Tissue engineering approaches reduce both donor site morbidity in graft procedures as well as the need for multiple surgeries. In this, biodegradable scaffolds are developed that hold cells; these scaffolds break down as new tissue forms and replaces the scaffold until full bodily function is regained. Synthetic polymers can offer tuneable mechanical and degradable characteristics alongside a low immunogenic response, which has made these materials a popular line of research as biodegradable scaffolds. This article seeks to summarise this field. Scaffold requirements, degradation factors and mechanisms, and common synthetic biodegradable polymers used in tissue scaffolding are covered, along with fabrication techniques. Specific examples of synthetic scaffolding polymers are explored for both bone and skeletal muscle to highlight the different desirable characteristics, hence the demands for each. Further research into new copolymer and scaffolding techniques will open new avenues to increased biocompatibility and clinical use, for which we recommend the creation of a comprehensive polymer database to store the vast library of synthetic polymer types and applications for future researchers.
Keywords: Tissue engineering, biodegradable polymers, synthetic biomaterials, bone and muscle regeneration, fabrication techniques of tissue scaffolds
Powered by decades of invention and improvement, synthetic polymer chemistry has progressed significantly since its discovery; this has resulted in the wide range of plastics that humans utilise every day. These polymers have highly functional properties, such as durability, stability and strength, making them well suited for numerous applications. The degradation of certain polymers is an exciting property that has implications in global waste management as well as biomedicine. Biodegradable polymers, which are inherently vulnerable to attack and degradation from the natural environment, are therefore an important aspect of the future of polymer chemistry. This degradability is highly desirable in numerous industries and, as such, these polymers have sparked particular interest in tissue engineering and medicine. The benefits of tissue engineering over traditional grafting procedures include the lack of donor site morbidity in autologous grafts as well as eliminating the need for recurrent or follow-up surgeries to remove non-degraded material (O’Brien, 2011). This field relies on the use of a scaffold, which aims to mimic the extracellular matrix (ECM), a structure that surrounds all cells, monitoring water and ion uptake, the diffusion of glucose and waste products, and protecting cells from external strain forces (Badylak, 2002; Flessner, 2001). Tissue engineering systems can be used in vitro – for example, in creating more accurate pathological models (Caddeo et al., 2017) – or they can be implanted into tissue defect sites as a treatment strategy. The biodegradable scaffold will hold cells in place and subsequently degrade at a controlled rate such that the cells replicate and create their own ECM to replace the scaffold, ultimately resulting in a fully functional regenerated tissue (Martina and Hutmacher, 2007). Biodegradable polymers show significant advantages over other materials used as tissue scaffolds primarily due to the reduced number of surgeries required for the removal of non-biodegradable scaffolds (Rezwan et al., 2006) and the minimal use of long-term immunosuppressant drugs (Martina and Hutmacher, 2007). However, biodegradable scaffolds need to be fine-tuned to ensure the correct balance between functional properties and biodegradation.
Ideal tissue scaffolds should therefore be extremely biocompatible in both scaffold and degraded form while providing suitable mechanical properties to withstand stress and support cells in vivo (Yang et al., 2001). Additionally, scaffolds should be highly porous and permeable, coupled with appropriate surface chemistry, to allow for the migration and attachment of cells into the scaffold while accommodating necessary nutrient exchange (Yang et al., 2001). These properties ensure the optimal function of tissue scaffolds in providing a suitable environment for cells to form functional tissue-like structures (O’Brien, 2011). The degradation rate of scaffolds should also be fine-tuneable to their particular applications to provide adequate structural properties throughout degradation, and eventually be replaced by the regenerated tissues. Moreover, the specific mechanical and compositional properties and requirements of a scaffold varies significantly with the type of tissue in question as well as with differences in patients, such as gender and age (Ge et al., 2008). A highly flexible and customisable design of scaffolds is therefore an important requirement when considering specific biodegradable polymers for implantation.
Although natural polymers such as collagen may provide superior biocompatibility while most closely resembling the in vivo environment, poor mechanical properties and difficulties with immunogenicity are still limitations (Alizadeh-Osgouei et al., 2019; Yang et al., 2001). However, natural polymers have the benefit of containing cell recognition and adhesion sites such as the arginine-glycine-aspartate (RGD) motif, which was initially recognised in natural polymers including fibrin and collagen (Kang et al., 2018). This mini-review will therefore focus on synthetic biodegradable scaffolds, with the benefits of composite materials incorporating natural polymers discussed in terms of application. Initially, this review will investigate the mechanisms of biodegradation as well as the specific chemistry of biodegradable polymers, which makes this process both possible and controllable. Subsequently, fabrication techniques, as well as examples of the application of specific biodegradable polymers in the fields of bone and skeletal muscle tissue engineering, will be reviewed to highlight the relevance of such materials in medical procedures. Figure 1 depicts a summary of these topics.

Before highlighting the use of biodegradable polymers in tissue engineering, it is important to discuss the degradation characteristics that make them attractive as biodegradable scaffolds. The two major mechanisms through which polymers are degraded in vivo are oxidative and hydrolytic biodegradation (Cortizo and Belluzo, 2017). The former relies on reactive radical molecules that are produced in vivo by the active process of phagocytic attack (Cortizo and Belluzo, 2017). Hydrolytic degradation, conversely, is a passive process classified as the cleavage of chemical bonds that are vulnerable to reaction with water (Cortizo and Belluzo, 2017). The relative insensitivity of synthetic polymers to enzymatic activity highlights passive hydrolysis as the major route of degradation in biological environments (Göpferich, 1996).
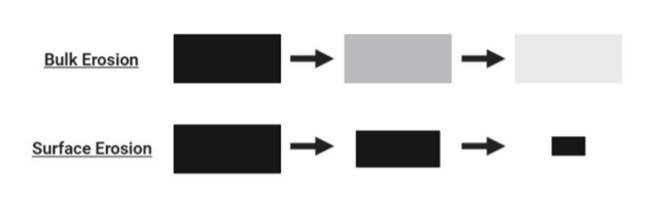
Hydrolytic degradation of polymers may occur via surface or bulk erosion. As the name suggests, surface erosion takes place only at the polymer surface, meaning that the macroscale polymeric scaffold becomes smaller while maintaining its geometry at a linear degradation rate. In contrast, bulk erosion proceeds throughout the polymer so that its size is conserved throughout degradation, although the degradation rate is no longer linear (Göpferich, 1996) (as depicted in Figure 2). Understanding which erosion type is predominant has important implications for tissue scaffolds and their intended applications (Göpferich et al., 1995; Göpferich, 1996; Pena et al., 2021). The hydrophobicity of scaffolds influences the rate of hydrolytic attack through impacting the osmosis of water into and throughout the polymeric scaffold. The pore size of polymeric scaffolds plays into this as larger pore sizes accommodate the ease of osmosis into the scaffold, in turn favouring bulk erosion (Odelius et al., 2011). Additionally, amorphous regions of polymers, being less densely packed and more accommodative of diffusion, are more vulnerable to passive hydrolysis by both surface and bulk erosion, and are thus eroded first in biological environments, resulting in the crystalline regions being intact for longer (Kroeze et al., 2009). It can subsequently be generalised that higher polymer crystallinity results in increased strength and stiffness as well as a slower degradation rate. The glass transition temperature (Tg) of polymers is another important aspect, especially when considering the mechanical requirements of scaffolds (Kroeze et al., 2009). Bone scaffolds, for example, typically require long-lasting mechanical properties, meaning that their Tg should be greater than body temperature, ensuring adequate mechanical stiffness while also preventing premature degradation (Kroeze et al., 2009).
Degradation kinetics are further significantly impacted by polymer molecular weight (Wuisman and Smit, 2006). Increasing molecular weight increases the formation of entanglements and secondary bonds between chains, which translates into increased binding forces between polymer chains, resulting in a decreased degradation rate (Wuisman and Smit, 2006). Dispersity, defined as the weight average molecular weight divided by the number average molecular weight (Mw/Mn), is a characteristic of polymers that can capture this information. Larger dispersity values highlight a smaller Mn – indicating a large number of smaller molecules, which are more easily degraded, therefore reducing the degradation time. Biodegradable polymers, therefore, require a lower dispersity, indicating less variance in polymer chain length, allowing for better predictions of the degradation rate (Wuisman and Smit, 2006) within decreased timescales that subsequently forgo issues such as inflammation and infection (Revati et al., 2015).
An attractive aspect of synthetic polymers for use in tissue engineering is the ability to tailor the degradation rate to suit specific applications. An example of how to achieve this is copolymerisation, in which the final product comprises blocks of different degradable polymers. This technique has been employed to fine-tune the degradation of poly-L-lactide (PLLA) (Agatemor and Shaver, 2013; Målberg, Höglund and Albertsson, 2011; Wanamaker, Tolman and Hillmyer, 2009), poly (ε-caprolactone) (PCL) (Xu et al., 2019) and polyurethane (Hong et al., 2010), to name a few. Other methods to tailor degradation rate include the addition of plasticisers (Burgos, Martino and Jiménez, 2013), surface modification (Cairns et al., 2011) and blending (Arias et al., 2014). Exploiting these techniques over the vast range of available polymers allows polymer degradation to be optimised to their function in tissue engineering.
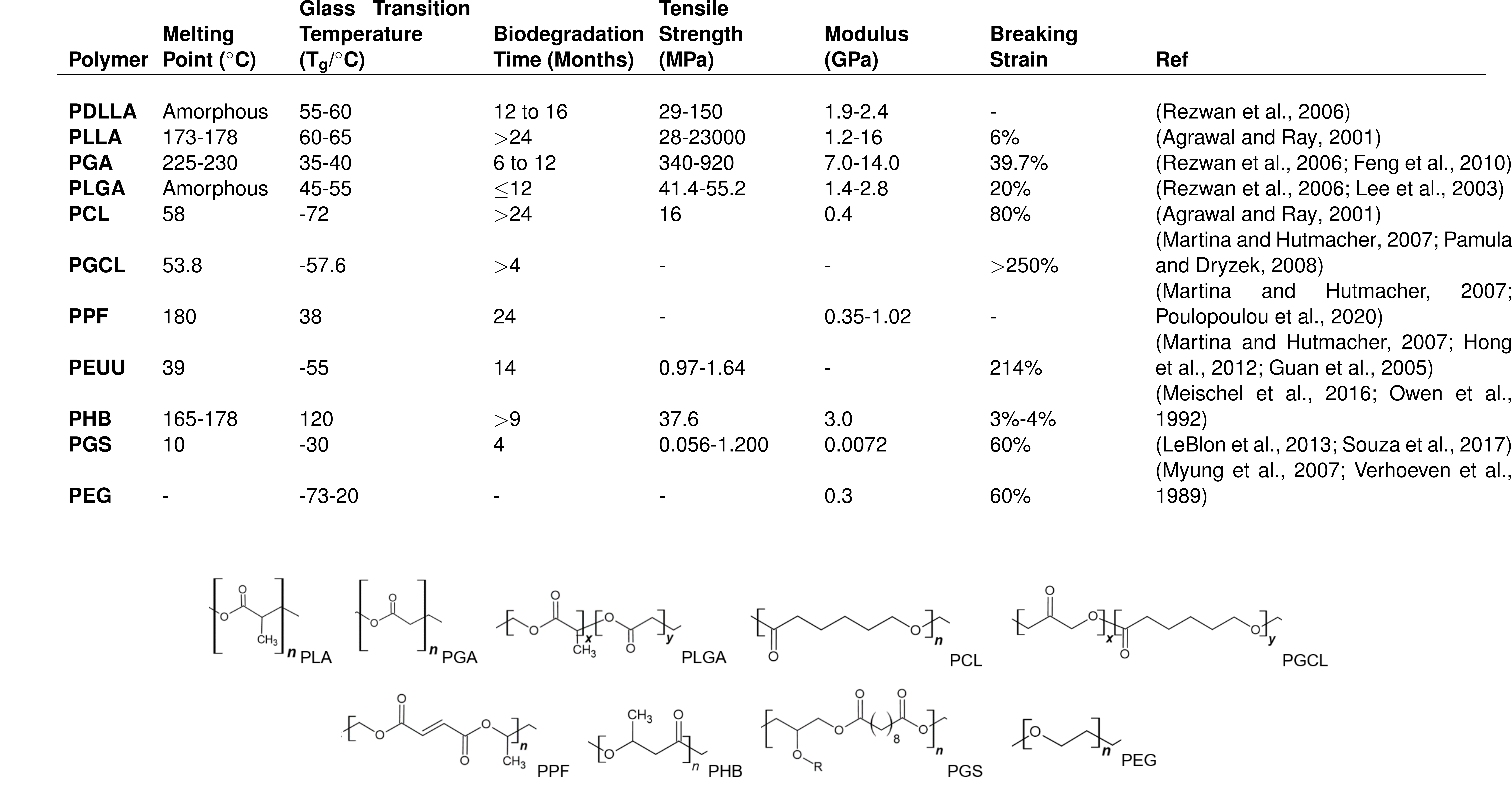
Numerous synthetic polymers possess the required properties of biodegradable scaffolds as outlined above, including polyurethanes, polyacetals and polyanhydrides (Rezwan et al., 2006). The most frequently used polymers for biodegradable tissue engineering, however, are synthetic aliphatic polyesters (Cortizo and Belluzo, 2017; Ge et al., 2008): predominantly poly (ε-caprolactone) (PCL), polylactic acid (PLA) (which exists in two optically isomeric forms (D and L) and a racemic form (DL)), and polyglycolic acid (PGA), as well as their copolymers (Rezwan et al., 2006). Coupled with their good biocompatibility and renewable production methods (Arora et al., 2018), these polymers are susceptible to hydrolytic degradation via de-esterification, and the resulting monomers (such as lactic acid and glycolic acid) are easily removed from the body, making them highly attractive as tissue scaffold materials (Rezwan al., 2006). Their biodegradable and bioresorbable properties have been well researched and used successfully in clinical products (Martina and Hutmacher, 2007).
However, there are inherent disadvantages to pure forms of these polymers that should be considered. The relatively weak cytocompatibility and the biological inertness of PLA are major disadvantages of its use in biodegradable tissue scaffolds (Al Tawil et al., 2018). Pure polymers often support decreased cellular interaction and tissue regeneration. To produce more biocompatible and biomimetic scaffolds, these polymers are often combined in block form with other polymers to customise their degradation and mechanical properties (Martina and Hutmacher, 2007). Other chemical modifications such as the addition of hyaluronan (Al Tawil et al., 2018), metallic nanoparticles (Ma et al., 2019) or ceramics (Göpferich et al., 1995) – especially hydroxyapatite (Shebi and Lisa, 2018) – have proven to improve the bioactivity of numerous polymers, allowing for more effective use in tissue engineering.
Other modifications include the blending of synthetic polymers with materials such as the aliphatic polyester group polyhydroxyalkanoates (PHA), which include poly-3-hydroxybutyrate (PHB) and poly-3-hydroxyoctanoate (PHO) (Chen and Wu, 2005). These are predominantly produced by microorganisms under artificially unbalanced conditions (Han et al., 2015), although it is possible to synthesise these polymers. PHB, in particular, can be built from various monomers, including BBL (Tschan et al., 2021), and propylene oxide and carbon monoxide to produce syndiotactic PHB (Winnacker 2019), which has a higher transitional melting temperature and lower crystallinity than its isotactic bacterial form (Turco et al., 2021). Unlike aliphatic polyesters, which are bulk-eroding, polyanhydrides and polyorthoesters are surface-eroding biomaterials. This allows them to keep their structural integrity for longer, and more steadily release drug payloads (Herwig and Dove, 2019). Polyanhydrides are the only FDA-approved surface-eroding biomaterial, although their complex synthesis and low mechanical strength have limited their wider use. Polyethylene glycol (PEG) is another more widely used polymer – a cross-linked hydrogel whose soft gel-like attribute gives it applications in drug delivery and wound repair; it degrades via different mechanisms depending on where on the body it is placed (Shi et al., 2021).
As demonstrated, there are many possible polymers to choose from. The most common synthetic biodegradable polymers in tissue engineering, and the properties that make them attractive, are summarised in Table 1. However, it is important to note that these are often copolymerised or modified for particular uses, altering the tabled values. A table that highlights all the available polymers as well as copolymers, composites and otherwise modified polymers, coupled with their different fabrication methods, properties and applications, would be too large to be feasible. However, consolidating this information into a summarised and straightforward database would enable researchers and industrial actors to understand the state of the art of certain polymers and allow faster decision making for future studies and industry. This database could be linked to the original research articles as well as reviews such as this, allowing individuals to delve deeper into the specifics after certain polymers or materials have already been selected.
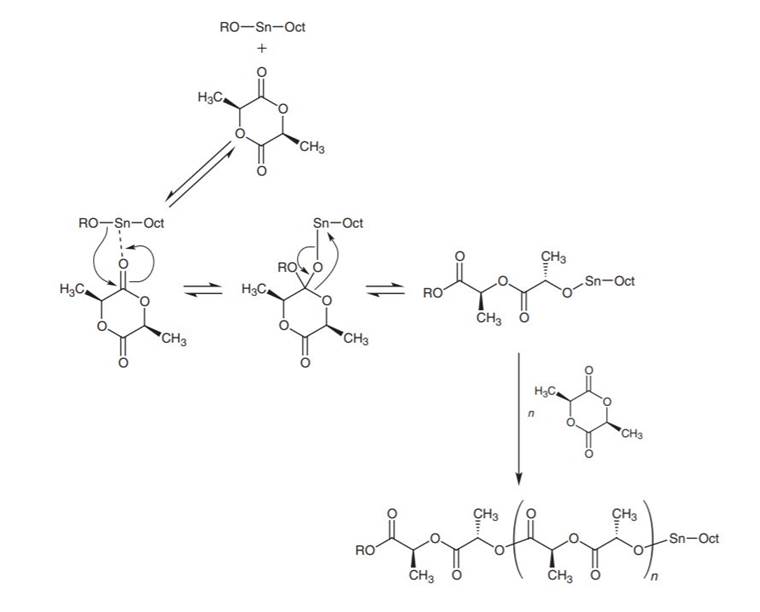
Before specific 3D scaffolds can be manufactured, the polymers themselves require production. Synthetic polymers can be produced via two mechanisms, (i) step-growth polymerisation of hydroxy-acid, or mixtures of diacid/diol monomers, or (ii) chain growth via ring-opening of cyclic monomers (Robert and Aubrecht, 2008). The former tends to be faster and produce more monomers of higher molecular weight (Billiet et al., 2009). However, for aliphatic polyesters, this is not the case (Fukushima and Nozaki, 2020), and so the latter technique is often favoured. Other benefits to chain-growth polymerisation include the elimination of extreme reaction conditions, elimination of unwanted by-products, and greater control over stereochemistry and molecular weights, resulting in higher-quality polymers (Jérôme and Lecomte, 2008; Van et al., 2016). It is therefore used widely for the production of PCL (Jérôme and Lecomte, 2008), PGA (Yamane et al., 2014), and PLA. However, ring-opening requires catalyst-initiators, one common example being tin (II) bis (2-ethylhexanoate), generally named Sn (Oct)2, which is used in the production of PLA in the presence of an alcohol (ROH) (Jérôme and Lecomte, 2008; Robert and Aubrecht, 2008). The mechanism of this reaction is detailed in Figure 3. The addition of heavy metal catalysts such as Sn (Oct)2 risks contamination during fabrication, increasing the costs and potential toxicity of the final product (Hu et al., 2018), and there is significant research to find metal-free catalysts that achieve the same reaction rate (Fukushima and Nozaki, 2020; Pappuru and Chakraborty, 2019; Tschan et al., 2021). Organocatalysts, in particular, have shown increasing promise for ring-opening polymerisation of racemic PLLA (Tschan et al., 2021).
Other polymers such as polyurethane (PU) and polyurethaneurea (PEUU) are more suited to production by step-growth polymerisation (Billiet et al., 2009), where it is cheaper and more efficient. They are mainly produced via reacting hexamethylene-diisocyanate (HMDI) with a diol, followed by subsequent reaction with other polymers such as PCL, resulting in a block polymer that can be degraded by de-esterification (Asefnejad et al., 2011; Goyker et al., 2021).
A third form of polymer synthesis is enzymatic polymerisation, which is under increasing study as a more environmentally friendly alternative to both step-growth and chain-growth methods (Douka et al., 2016). Here, synthetic polymers such as PCL are mixed with immobilised enzymes such as ionic-liquid-coated lipases, which have been isolated from bacterial culture and placed in a solvent solution (Douka et al., 2016). This technique can produce high molecular masses of polymers such as polyesters (Zhao, 2019), although reaction optimisation and commercial viability are still being explored.
Multiple monomers are often incorporated into the fabrication process. Block polymerisation acts to combine the properties of its constituent homopolymer sections based on their mixture ratio (Dong et al., 2017). In contrast, copolymerisation of multiple monomers is widely used to create materials with novel properties, with the potential for lower stiffness, increased crystallinity, or higher degradation than any one homopolymer (Middleton and Tipton, 2000).
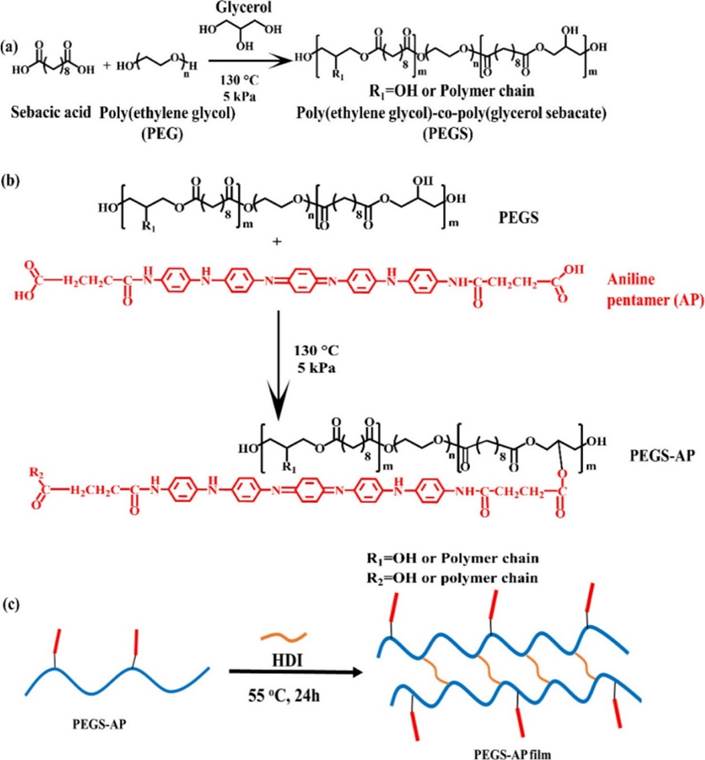
Often these production methods produce simple pre-polymer forms, and their final structure is reached with the addition of other polymer blocks and/or side chains, such as in Figure 4. These steps allow further modification and control of polymer properties at the atomic level (Dong et al., 2017).
Once these polymers have been produced, they can be processed into scaffold structures to fulfil their function in tissue engineering. The technique used for scaffold production can significantly influence its structure and functionality in vivo. There are many methods, with the most common including solvent casting, gas foaming, electrospinning, particulate leaching as well as additive manufacturing, with each resulting in a unique architecture and functionality (Agrawal and Ray, 2001; Alizadeh-Osgouei et al., 2019).
Particulate leaching involves the addition of soluble particles (often NaCl crystals) of a set size and adding them to the polymer during its formation. The particles are later dissolved in deionised water, leaving behind a network of porous holes (Haider et al., 2020). A 3D scaffold can be formed by laminating layers of individually leached sheets (Agrawal and Ray, 2001). Particulate leaching is a relatively cheap and easy technique, but as pore interconnectivity is determined only by the size and quantity of the particles added, it lacks precise structural control (Deb et al., 2018; Haider et al., 2020). It is suited to scaffolding structures of extremely high porosity (and therefore lower load-bearing capabilities), such as endothelial tissue, as this guarantees good pore interconnectivity (Haider et al., 2020).
Electrospinning is one of the most widely researched scaffold formation techniques (Haider et al., 2020). It creates electrically active fibres by blending a biodegradable polymer such as PCL with a conductive polymer and ejecting the solution from a needle under high voltage. Electrospun nanofibrous materials have controllable porosities and large surface area-volume ratios, and the alignment of fibrilstuneable with the addition of an external magnetic field (Chen et al., 2013). This technique is highly successful, but requires the optimisation of many parameters, including applied voltage, solution concentration and humidity of the system (Haider et al., 2020). Currently, the main challenge is that a significant component of the scaffold must be a conductive polymer, which can affect its mechanical properties (Chen et al., 2013).
Advances in additive manufacturing technologies have also provided exciting avenues for scaffold production. Techniques such as stereolithography, fused-deposition modelling (FDM) and selective laser sintering allow size, porosity and geometry to be tightly controlled across the scaffold (Shick et al., 2019). In FDM, thermoplastic polymers such as PCL and PLA are melted and extruded to produce layer-by-layer depositions from a computer-aided-design model. It enables the production of complex porous scaffolds of tuneable and accurate dimensions (Ogden et al., 2014; Temple et al., 2014; Wei et al., 2012). Cross-linking polymers such as PPF have also been employed in 3D printing methods to similar success (Wei et al., 2012). This method, guided by patient CT scans, can be used to create personalised scaffolds, and has proven to seed cells resulting in successful bone formation (Temple et al., 2014). Its precision and patient-specific potential make optimisation of additive manufacturing techniques highly desirable, and this research area is very active (Ahangar et al., 2019; Shick et al., 2019; Tan et al., 2020). Finally, once the polymers have been formed and the scaffold has been built, the structure can then receive further biocompatibility modification by coating the fabricated scaffold with a thin layer of degradable bioactive material (such as hydroxyapatite or magnesium) via plasma spraying or bio-ink printing (Alizadeh-Osgouei et al., 2019).
Numerous tissue types exist in the body, each having specific structural properties and ECM composition. Customisation of scaffold properties is extremely useful when considering the tissue type needing to be replaced. Bones, which provide support and protection for the body (Su et al., 2019), deliver increased mechanical support and have a larger ECM:cell ratio consisting of high quantities of collagen (Badylak, 2002). Conversely, skin and muscles do not require such mechanical strength, but instead need to stretch, resulting in an increased proportion of elastin in the ECM (Khalili et al., 2019). Tendons and ligaments act as elastic springs to transmit forces between muscle and bone, stretching and recoiling to improve movement efficiency. These need both high mechanical and tensile strength, achieved with high levels of aligned collagen fibrils (Beldjilali-Labro et al., 2018; Sensini et al., 2021). It is evident from this small overview that the scaffolding requirements for each tissue type differ (Martina and Hutmacher, 2007), and these must be considered when selecting polymers and fabrication methods.
This section looks at the specific requirements and applications of biodegradable synthetic polymers in two tissue types chosen for their contrast: bone and skeletal muscle. Studies into bone biomaterials have had high success, with clinical trials and a range of products now commercially available (Alizadeh-Osgouei et al., 2019; Pereira et al., 2020; Zeng, et al., 2018). Conversely, Skeletal muscle has, to the authors knowledge, no currently available scaffold that has passed clinical trials. These differences in patient availability have their roots in tissue complexity, but they are also linked to funding availability and the suitability of currently available approved polymers to their requirements (Freedman and Mooney, 2019; Williams, 2019). Here, we will focus on synthetic scaffolding techniques used or under research in the two sub-fields.
The unique mechanical requirements of bone-tissue scaffolds result in an intricate interplay between mechanical support and degradation time, and these must be controlled to allow for regenerated bone to replace the support lost from the scaffold. A porosity between 80 to 90 per cent (Roy et al., 2003), as well as a pore size larger than 300 μm (Ge et al., 2008; Karageorgiou and Kaplan, 2005), are ideal for bone regeneration or osteoinductivity (Ge et al., 2008). This may be enhanced by the incorporation of osteoinductive – or growth – factors that can be released upon degradation (Chen and Mooney, 2003; Ge et al., 2008). Natural bone is a composite material composed primarily of collagen, a polymer, as well as the inorganic ceramic apatite (Yang et al., 2018). Using composite scaffolds comprising both polymeric and inorganic phases therefore mimics this natural environment and may prove beneficial in achieving regeneration. The ideal properties for bone-tissue engineering scaffolds have been encompassed by numerous polymers and polymer composites to obtain clinical-grade scaffolds, which have proven successful in bone regeneration and have resulted in commercial products. Due to being granted US FDA approval, aliphatic polyesters such as PLA, PGA and PCL have been employed to the largest extent. Particular research papers that fast-tracked commercial development will be outlined below, followed by examples of some currently available products.
Yang et al. (2018) used a PLGA electrospun scaffold with incorporated silica nanoparticles, resulting in heightened bone nodule formation and collagen secretion, proving that this particular scaffold stimulates osteogenic differentiation in vitro. Another PLGA composite, functionalised with the osteoinductive bone morphogenetic protein 2 (BMP-2)-like peptide, has successfully been employed to repair a critical-sized cranial defect in a rat model (Zhang et al., 2019). The mechanical similarities of the PLGA composite used in this study, coupled with the demonstration of inducing osteogenic differentiation as well as bone formation in vivo, highlight this as an attractive scaffold for use in human bone-tissue engineering. PLA, together with a metal core, has been used as a biodegradable bone graft for hip replacement surgery, proving to be mechanically stable and biocompatible for successful bone regeneration (Lagoa et al., 2008).
Other research avenues have incorporated inorganic material into scaffolds to enhance biomimicry and improve bone-tissue regeneration. A PLA/hydroxyapatite composite has shown in vivo success as a bone-filling scaffold as far back as 1986 (Higashi et al., 1986). More recently, a PCL/hydroxyapatite composite supported the growth and differentiation of bone marrow mesenchymal stem cells (Chen and Chang, 2011). Beyond biomimicry, the incorporation of hydroxyapatite in these studies eliminates the limitations of brittleness and low mechanical strength associated with pure hydroxyapatite (Zhang et al., 2021). Research into polymeric composites containing hydroxyapatite is ongoing, and recent studies have been able to use 3D printing to increase the hydroxyapatite content in a PLA composite without significantly impacting the mechanical properties of the scaffold (Dubinenko et al., 2020; Zhang et al., 2021). Despite the benefit of this scaffold both in vitro and in vivo in the former study, issues remained with increased acidity during degradation of PLA, leading to potential inflammation. Although increased hydroxyapatite aided this, further research is still required before the large-scale clinical uptake of this scaffold.
Due to the benefit of containing cell-binding RGD sites, natural polymers may be incorporated into synthetic polymeric scaffolds or used as coatings with the goal of aiding cell adhesion. This benefit has been realised using a collagen coating, which positively influenced cellular attachment and differentiation in PLGA (Wu et al., 2006) and PLLA (Zhang et al., 2013). Another study used a small intestinal submucosa (SIS)/PLA scaffold together with the sustained release of a BMP2-related peptide, termed P28, to enhance bone regeneration (Xiong et al., 2020). The combination of the collagen I and glycosaminoglycan containing SIS together with PLA achieved a highly biomimetic scaffold with tuneable degradation coupled with tuneable bone-tissue formation in vivo. Numerous additional studies involving many polymer types, fabrication methods, applications and degradation times have been demonstrated, the details of which can be found in review articles (Narayanan et al., 2016; Prasad, 2021; Teoh et al., 2019).
Studies like those mentioned above prove the potential use of specific polymers in bone regeneration. This has propelled numerous clinical trials and the creation of commercial products. LactoSorb® is a PLA-based product line of screws and implants from Zimmer Biomet, which have applications in craniofacial surgeries (Zimmer Biomet, 2019). These products have a predictable degradation of approximately 12 months (Eppley and Reilly, 1997), and show low inflammation and infection rates (Goldstein et al., 1997). Many other PLA-based products, such as Rapidsorb™ and Biocryl®, are commercially available and are summarised in Narayanan et al. (2016). The commercial success of other polymer types is less pronounced, although numerous clinical trials are currently underway, including the use of 3D-printed PCL scaffolds in dental surgery (ClinicalTrials.gov, 2019).
Muscle contains specific fibril lengths and alignments to allow muscle to effectively distribute force along the tissue (Corona and Greising, 2016). When building new tissue, the stem cells in skeletal muscle known as satellite cells (Wang and Rudnicki, 2012) proliferate and differentiate into multi-nucleated myoblasts (Rizzi et al., 2012), which then meld together into myotubes. A skeletal muscle scaffold must be able to direct cell migration and growth effectively to create these parallel, highly organised fibres, and so encouragement of satellite cell migration into the scaffold is vitally important (Corona and Greising, 2016). Tissue scaffolding of skeletal muscle is made more complex by the fact that the architecture of functioning muscle is mainly controlled mechanically. A static scaffold is not able to give these physical growth cues, so the myotubes grow in a randomly-oriented way (Chen et al., 2013). This makes cultivated skeletal muscle very poor at force bearing in vivo (Gentile et al., 2014; Mase et al., 2010). In order to avoid this random orientation of myoblast formation, the scaffold must be subjected to a rhythmic electrical/mechanical component to mimic actual muscle use (Rizzi et al., 2012).
One major line of research has been looking into the incorporation of conductive polymers into the skeletal muscle scaffold (Dong et al., 2020). As well as initiating muscle contraction, electrical stimulation has been shown to align myoblasts parallel to the direction of electric field vectors (Chen et al., 2019), and may provide a simple and cost-effective method to ensure both alignment and contractile ability. Electrospinning, which requires a conductive polymer component to function, is able to produce controlled alignment of polymer fibres, and is therefore highly suited to the task. Chen et al. (2013) used an electrospinning technique with a combination of PCL and polyaniline (PANi) to produce such an aligned fibrous polymer scaffold. They found increased cell proliferation and myotube fusion in in vitro mouse myoblasts compared to a non-aligned PCL/PANi mix. Jun et al. gained similar results using a blend of poly (L-lactide-co-epsilon-caprolactone) (PLCL) and PANi (Jun et al., 2009), which combined the rigidity and brittle nature of PANi with the highly elastic PLCL, creating a more suitable scaffold than either pure polymer. A 3:7 PANi–PLCL ratio was able to achieve 170 per cent strain, greater than the strain exhibited by skeletal muscle. However, despite its biocompatibility, PANi does not degrade (Chen et al., 2013; Dong et al., 2017), and so more experimentation into its polymer structure is needed before it can give the properties a biodegradable scaffold requires.
Another route into skeletal muscle scaffolding is the use of hydrogels. PEG is a highly biocompatible hydrogel that has been approved by the FDA ‘for internal consumption’ (Choi et al., 2019). Its properties are easily altered by changing its Mn, water:polymer ratio, and cross-linking density (Almany and Seliktar, 2005). Its derivative, PEGDA (produced by substituting its terminal hydroxyl groups with acrylate), is able to gelate from a liquid to a solid state under UV light after cells have been suspended within it (Choi et al., 2019; Fuoco et al., 2014). Its scaffold fabrication can therefore occur after myoblasts have grown into it, allowing mechanical signalling to define muscular tissue structure rather than a prefabricated scaffold shape. When combined with a biological cell-adhesive backbone such as fibrinogen, PEG creates a scaffold with both controllable physical characteristics and cell-signalling capabilities (Fuoco et al., 2014; Rizzi et al., 2012), promoting skeletal muscle regeneration and blood vessel growth in vitro (Fuoco et al., 2014). PEG can also be functionalised with maleimide groups, which enable it to hold stem cells and bind to patient tissue, used by Han et al. (2015) in vivo as an injectable scaffolding cell-delivery system. Dong et al. (2017) were able to combine the benefits of hydrogels with the abilities of conductive polymers. They combined polyglycerol sebacate (PGS), a highly elastic but poorly hydrophilic polymer, with the highly hydrophilic PEG (Dong et al., 2017). The resulting polymer PEGS had aniline pentamer (AP) side chains added via esterification for conductivity. PEGS films had fatigue-free mechanical properties, and promoted myoblast proliferation. This conductive, flexible hydrogel has the potential to allow both electrical and mechanical stimulus to direct tissue formation, making it a good candidate for further study.
A more recent advance into biodegradable skeletal muscle is the combined use of aliphatic polyesters with polyurethanes. ‘Thermoplastic PU and PEUU copolymers’ (TPUs) (Daemi et al., 2016; Goyker et al., 2021) are prepared by combining hydrophobic PCL and hydrophilic PEG copolymerised ‘soft’ segments, and PU ‘hard’ segments. The tuneability of synthetic polymer production allows easy tailoring of the synthetic process to optimise the soft and hard segment ratios to suit TPU for skeletal muscle scaffolding. Goyker et al. (2021) described a 3D-printed scaffold of aligned TPU filaments ‘soft yet durable, strong, elastic, and hydrophilic’. When tested in vivo, after four weeks of implantation, they found myoblast regeneration and some capillary formation in the implant site, with an 86 per cent recovery of function (Goyker et al., 2021).
The unique biocompatible and biodegradable requirements of tissue scaffolds and the complexity of their interactions within the human body gives this area of study distinct challenges. Not only must a scaffold function and degrade appropriately, but it must also do so for the right tissue type, each of which has its own mechanical and morphological requirements. Polymer degradation kinetics must be controlled to avoid voids and inflammation during healing, and although a low dispersity is optimal, other factors such as crystallinity, Tg and strain values are all dependent on tissue type. Well-researched polymers such as PLA and PCL are often used in conjunction with other less degradable but more tissue-compatible polymers, either in block form or as a modification to combine their properties into a scaffold more suitable for its function than either polymer in its pure state. This control over scaffold properties and mechanics is further enhanced via the multitude of fabrication techniques that a polymer may be formed by to create the 3D scaffold the body requires, altering porosity and fibre organisation to control cell migration and growth. Bone, with its requirement for high porosity and stress values, has produced PLA-based bone scaffolds that have been used successfully in clinical trials. Muscle, requiring a conductive and elastic scaffold able to experience mechanical loading, has only produced success in vitro so far with PCL and PEG-based polymer scaffolding.
The low number of FDA- and MHRA-approved biodegradable scaffolding polymers has limited clinical research to only a handful of polymers, and although their properties have been widely researched, they are not optimal in their pure states for scaffolding. Research into new polymer structures is limited to in vitro experimentation, and the rigorous testing required before clinical trials can be undergone means that even a perfect scaffold is still a decade away from use in the population. Despite this, great advances have been made in understanding how to control the growth of cells using fabricated polymer structures, and in providing optimum conditions for this process.
One great advantage of synthetic polymers is their tuneability – of both mechanical and degradation characteristics (Reddy et al., 2021; Williams, 2019). However, this tuneability, alongside co- and block polymerisation and variations in scaffolding techniques, has produced a field with a vast number of potential polymer and scaffolding structures of which this review has only scratched the surface. The ideal next step for the field would be the creation of a publicly available database of known scaffolding polymers, with their properties and the effects of different fabrication techniques and copolymer additions available to potential researchers. Such a comprehensive comparison of these polymers would help refine and channel future study.
The field of tissue engineering using degradable scaffolds is still in its infancy. There are many hurdles to overcome before this technology is in regular clinical use. But further investigation into the role of the ECM in cell growth, alongside the testing of copolymers and novel fabrication techniques, will only further enhance the possibilities of this promising field of study.
Author contributions: HM and EH contributed equally in doing the research and writing the paper. AISN supervised as part of the module NSC3007 Macromolecular and Supramolecular Chemistry and reviewed the document. HM would like to thank the Erasmus + programme of the European Commission for the funding that enabled the placement at the University of Exeter.
Figure 1: Overview of synthetic biodegradable polymers and their application in tissue engineering. Figure is authors’ own.
Figure 2: Schematic of surface and bulk erosion. Figure is authors’ own.
Figure 3: Ring-opening chain-growth polymerisation to produce PLA. (Reprinted with permission from Robert and Aubrecht (2008), Copyright (2008) American Chemical Society.)
Figure 4: Formation of a PEGS-AP polymer. (Reprinted with permission from Dong, Zhao, Guo and Ma (2017), Copyright (2017) American Chemical Society.)
Table 1: Summary of commonly used synthetic polymers and a schematic depiction of their chemical structure.
Agatemor, C. and M. P. Shaver (2013), ‘Tacticity-induced changes in the micellization and degradation properties of poly (lactic acid)-block-poly (ethylene glycol) copolymers’, Biomacromolecules, 14 (3), 699–708
Agrawal, C. and R. Ray (2001), ‘Biodegradable polymeric scaffolds for musculoskeletal tissue engineering’, Journal of Biomedical Materials Research, 55 (2), 141–50
Ahangar, P., M. Cooke, M. Weber and D. Rosenzweig (2019), ‘Current biomedical applications of 3D printing and additive manufacturing’, Applied Sciences, 9,1–23
Al Tawil, E., A. Monnier, Q. Nguyen and B. Deschrevel (2018), ‘Microarchitecture of poly (lactic acid) membranes with an interconnected network of macropores and micropores influences cell behavior’, European Polymer Journal, 105, 370–88
Alizadeh-Osgouei, M., L. Yuncang and C. Wen (2019), ‘A comprehensive review of biodegradable synthetic polymer-ceramic composites and their manufacture for biomedical applications’, Bioactive Materials, 4, 22–36
Almany, L. and D. Seliktar (2005), ‘Biosynthetic hydrogel scaffolds made from fibrinogen and polyethylene glycol for 3D cell cultures’, Biomaterials, 26 (15), 2467–77
Arias, V., A. Höglund, K. Odelius and A. C. Albertsson (2014), ‘Tuning the degradation profiles of poly (l-lactide)-based materials through miscibility’, Biomacromolecules, 15 (1), 391–402
Arora, B., R. Bhatia and P. Attri (2018), ‘Bionanocomposites: Green materials for a sustainable future’, in Hussain, C. M. and A. K. Mishra (eds), New Polymer Nanocomposites for Environmental Remediation, Elsevier Inc., pp 699–712
Asefnejad, A., M. Khorasani A. Behnamghader B. Farsadzadeh and S. Bonakdar (2011), ‘Manufacturing of biodegradable polyurethane scaffolds based on polycaprolactone using a phase separation method: Physical properties and in vitro assay’, International Journal of Nanomedicine, 6, 2375–84
Badylak, S. (2002), ‘The extracellular matrix as a scaffold for tissue reconstruction’, Seminars in Cell and Developmental Biology,13 (5), 377–83
Beldjilali-Labro, M., A. Garcia F. Farhat F. Bedoui J. F. Grosset M. Dufresne and C. Legallais (2018), ‘Biomaterials in tendon and skeletal muscle tissue engineering: current trends and challenges’, Materials, 11, 1–49
Billiet, L., D. Fournier and F. Prez (2009), ‘Step-growth polymerization and “click” chemistry: The oldest polymers rejuvenated’, Polymer, 50, 3877–86
Burgos, N., V. P. Martino and A. Jiménez (2013), ‘Characterization and ageing study of poly (lactic acid) films plasticized with oligomeric lactic acid’, Polymer Degradation and Stability, 98 (2), 651–658
Caddeo, S., M. Boffito and S. Sartori (2017), ‘Tissue engineering approaches in the design of healthy and pathological in vitro tissue models’, Frontiers in Bioengineering and Biotechnology, 5, 1–22
Cairns, M.-L., G. R. Dickson J. F. Orr D. Farrar K. Hawkins and F. J. Buchanan (2011), ‘Electron-beam treatment of poly (lactic acid) to control degradation profiles’, Polymer Degradation and Stability, 96 (1), 76–83
Chen, J. and Y. Chang (2011), ‘Preparation and characterization of composite nanofibers of polycaprolactone and nanohydroxyapatite for osteogenic differentiation of mesenchymal stem cells’, Colloids and surfaces B, Biointerfaces, 86 (1), 167–75
Chen, R. and D. Mooney (2003), ‘Polymeric growth factor delivery strategies for tissue engineering’, Pharmaceutical Research, 20 (8), 1103–12.
Chen, G.-Q. and Q. Wu (2005), ‘The application of polyhydroxyalkanoates as tissue engineering materials’, Biomaterials, 26 (33), 6565–78
Chen, M.-C., Y. -C. Sun and Chen, Y.-H. (2013), ‘Electrically conductive nanofibers with highly oriented structures and their potential application in skeletal muscle tissue engineering’, Acta Biomaterialia, 9 (3), 5562–72
Chen, C., X. Bai, Y. Ding and I. S. Lee (2019), ‘Electrical stimulation as a novel tool for regulating cell behavior in tissue engineering’, Biomaterials Research, 23 (25), 1–12
Choi, J., K. Yong, J. Choi and A. Cowie (2019), ‘Recent advances in photo-crosslinkable hydrogels for biomedical applications’, BioTechniques, 66 (1), 40–53
ClinicalTrials.gov (2019), ‘3D printed scaffold device for ridge preservation after tooth extraction’, available at https://clinicaltrials.gov/ct2/show/NCT03735199#wrapper, accessed 15 November 2020
Corona, B. and S. Greising (2016), ‘Challenges to acellular biological scaffold mediated skeletal muscle tissue regeneration’, Biomaterials, 104, 238–46
Cortizo, M. and M. Belluzo (2017), ‘Biodegradable polymers for bone tissue engineering’, in Goyanez, S. N. and N. B. D’Accorso (ed.), Industrial Applications of Renewable Biomass Products: Past, Present and Future, Springer International Publishing, pp 47–74
Daemi, H., S. Rajabi-Zeleti, H. Sardon, M. Barikani, A. Khademhosseini and Baharvand, H. (2016), ‘A robust super-tough biodegradable elastomer engineered by supramolecular ionic interactions’, Biomaterials, 84, 54–63
Deb, P., A. Deoghare, A. Borah, E. Barua and S. Das Lala (2018), ‘Scaffold development using biomaterials: A review’, Materials Today: Proceedings, 5, 12909–19
Dong, R., X. Zhao, B. Guo and P. Ma (2017), ‘Biocompatible elastic conductive films significantly enhanced myogenic differentiation of myoblast for skeletal muscle regeneration’, Biomacromolecules, 18 (9), 2808–19
Dong, R., P. Ma and B. Guo (2020), ‘Conductive biomaterials for muscle tissue engineering’, Biomaterials, 229, 1–20
Douka, A., S. Vouyiouka, L. M. Papaspyridi and C. Papaspyrides (2016), ‘A review on enzymatic polymerization to produce polycondensation polymers: The case of aliphatic polyesters, polyamides and polyesteramides’, Progress in Polymer Science, 79, 1–25
Dubinenko, G. E., A. L. Zinoviev, E. N. Bolbasov, V.T. Novikov and Tverdokhlebov, S. I. (2020), ‘Preparation of Poly (L-lactic acid)/Hydroxyapatite composite scaffolds by fused deposit modeling 3D printing’, Materials Today: Proceedings, 22, 228–234
Eppley, B. and M. Reilly (1997), ‘Degradation characteristics of PLLA-PGA bone fixation devices’, The Journal of craniofacial surgery, 8 (2), 116–20
Feng, C., Y. -M. Xu, Q. Fu, W. -D. Zhu, L. Cui and J. Chen(2010), ‘Evaluation of the biocompatibility and mechanical properties of naturally derived and synthetic scaffolds for urethral reconstruction’, Journal of Biomedical Materials Research, 94A (1), 317–25
Flessner, M. (2001), ‘The role of extracellular matrix in transperitoneal transport of water and solutes’, Peritoneal Dialysis International, 21 (3), 24–29
Freedman, B. and D. Mooney (2019), ‘Biomaterials to mimic and heal connective tissues’, Advanced Materials, 31, 1–27
Fukushima, K. and K. Nozaki (2020), ‘Organocatalysis: A paradigm shift in the synthesis of aliphatic polyesters and polycarbonates’, Macromolecules, 53 (13), 5018–22
Fuoco, C., E. Sangalli, R. Vono, S. Testa, B. Sacchetti and M. Latronico (2014), ‘3D hydrogel environment rejuvenates aged pericytes for skeletal muscle tissue engineering’, Frontiers in Physiology, 5 (203), 1–8
Ge, Z., Z. Jin and T. Cao (2008), ‘Manufacture of degradable polymeric scaffolds for bone regeneration’, Biomedical materials, 3 (2), 1–11
Gentile, N., K. Stearns, E. Brown, J. Rubin, M. Boninger and C. Dearth (2014), ‘Targeted rehabilitation after extracellular matrix scaffold transplantation for the treatment of volumetric muscle loss’, American Journal of Physical Medicine & Rehabilitation, 93 (11), 79–87
Goyker, S., E. Yilgor, I. Yilgor, E. Berber, E. Vrana, K. Orhan, Y. Monsef, O. Guvener, M. Zinnuroglu, C. Oto and P. Huri (2021), ‘3D printed biodegradable polyurethaneurea elastomer recapitulates skeletal muscle structure and function’, ACS Biomaterials Science and Engineering, 7, 5189–205
Goldstein, J., F. Quereshy and A. Cohen (1997), ‘Early experience with biodegradable fixation for congenital pediatric craniofacial surgery’, The Journal of Craniofacial Surgery, 8 (2), 110–15
Göpferich, A., D. Karydas and R. Langer (1995), ‘Predicting drug release from cylindric polyanhydride matrix discs’, European Journal of Pharmaceutics and Biopharmaceutics, 41 (2), 81–87
Göpferich, A. (1996), ‘Mechanisms of polymer degradation and erosion’, Biomaterials17 (2), 103–14.
Guan, J., K. Fujimoto, M. Sacks and W. Wagner (2005), ‘Preparation and characterization of highly porous, biodegradable polyurethane scaffolds for soft tissue applications’, Biomaterials, 18 (26), 3961–71
Haider, A., S. Haider, M. Kummara, T. Kamal, A. Alghyamah, F. Iftikhar, B. Bano, N. Khan, M. Afridi, S. Han, A. Alrahlah, and R. Khan (2020), ‘Advances in the scaffolds fabrication techniques using biocompatible polymers and their biomedical application: A technical and statistical review’, Journal of Saudi Chemical Society, 24, 186–215
Han, J., L. -P. Wu, J. Hou, D. Zhao and H. Xiang (2015), ‘Biosynthesis, Characterization, and hemostasis potential of tailor-made poly (3-hydroxybutyrate-co-3-hydroxyvalerate) produced by haloferax mediterranei’, Biomacromolecules, 16 (33), 578–88
Herwig, G. and A. Dove (2019), ‘Synthesis of rapidly surface eroding polyorthoesters and polyacetals using thiol−ene click chemistry’, American Chemical Society Macro Letters, 8, 1268–74
Higashi, S., T. Yamamuro, T. Nakamura, Y. Ikada, S. Hyon and K. Jamshidi (1986), ‘Polymer-hydroxyapatite composites for biodegradable bone fillers’, Biomaterials, 7 (3), 183–87
Hong, Y., J. Guan, K. L. Fujimoto, R. Hashizume, A. L. Pelinescu and W. R. Wagner (2010), ‘Tailoring the degradation kinetics of poly (ester carbonate urethane)urea thermoplastic elastomers for tissue engineering scaffolds’, Biomaterials, 31 (15), 4249–58
Hong, Y., K. Takanari, N. Amoroso, R. Hashizume, E. Brennan-Pierce and J. Freund (2012), ‘An elastomeric patch electrospun from a blended solution of dermal extracellular matrix and biodegradable polyurethane for rat abdominal wall repair’, Tissue Engineering Part C Methods, 18 (2), 122–32
Hu, L., C. J. Zhang, H. L. Wu, J. L. Yang, B. Liu, H.Y. Duan and X. H. Zhang (2018), ‘Highly active organic lewis pairs for the copolymerization of epoxides with cyclic anhydrides: Metal-free access to well-defined aliphatic polyesters’, Macromolecules, 51, 3126–34
Jun, I., S. Jeong and H. Shin (2009), ‘The stimulation of myoblast differentiation by electrically conductive sub-micron fibers’, Biomaterials, 30 (11), 2038–47
Jérôme, C. and P. Lecomte (2008), ‘Recent advances in the synthesis of aliphatic polyesters by ring-opening polymerization’, Advanced Drug Delivery Reviews, 60 (9), 1056–76
Kang, Z., Y. Wang, J. Xu, G. Song, M. Ding, H. Zhao and J. Wang, J. (2018), ‘An RGD-containing peptide derived from wild silkworm silk fibroin promotes cell adhesion and spreading’, Polymers, 10 (11), 1193
Karageorgiou, V. and D. Kaplan (2005), ‘Porosity of 3D biomaterial scaffolds and osteogenesis’, Biomaterials, 26 (27), 5474–91
Khalili, S., S. Khorasani, S. Razavi, B. Hashemibeni and A. Tamayol (2019), ‘Nanofibrous scaffolds with biomimetic composition for skin regeneration’, Applied Biochemistry and Biotechnology, 187 (4), 1193–203
Kroeze, R., M. Helder, L. Govaert and T. Smit (2009), ‘Biodegradable polymers in bone tissue engineering. Materials’, Biomaterials, 3 (2), 833–56
Lagoa, A., C. Wedemeyer, M. von Knoch, F. Löer and M. Epple (2008), ‘A strut graft substitute consisting of a metal core and a polymer surface’, Journal of Materials Science: Materials in Medicine, 19 (1), 417–24
LeBlon, C., R. Pai, C. Fodor, A. Golding, J. Coulter and S. Jedlicka (2013), ‘In vitro comparative biodegradation analysis of salt-leached porous polymer scaffolds’, Journal of Applied Polymer Science, 128 (5), 2701–12
Lee, S.-H., B. -S. Kim, S. Kim, S. Choi, S. Jeong and K. IK (2003), ‘Elastic biodegradable poly (glycolide-co-caprolactone) scaffold for tissue engineering’, Journal of Biomedical Materials Research, 66A (1), 29–37
Lund, D. and D. Cornelison (2013), ‘Enter the matrix: Shape, signal and superhighway’, The FEBS Journal, 280 (17), 4089–99
Ma, S., Z. Wang, Y. Guo, P. Wang, Z. Yang and L. Han (2019), ‘Enhanced osteoinduction of electrospun scaffolds with assemblies of hematite nanoparticles as a bioactive interface’, International Journal of Nanomedicine, 14, 1050–68
Målberg, S., A. Höglund and A. C. Albertsson (2011), ‘Macromolecular design of aliphatic polyesters with maintained mechanical properties and a rapid, customized degradation profile’, Biomacromolecules, 12 (6), 2382–88
Martina, M. and D. Hutmacher (2007), ‘Biodegradable polymers applied in tissue engineering research: A review’, Polymer International, 56 (2), 145–57
Mase, VJ, J. Hsu, S. Wolf, J. Wenke, D. Baer and J. Owens (2010), ‘Clinical application of an acellular biologic scaffold for surgical repair of a large, traumatic quadriceps femoris muscle defect’, Orthopedics, 33 (7), 1–20
Meischel, M., J. Eichler, E. Martinelli, U. Karr, J. Weigel and G. Schmöller (2016), ‘Adhesive strength of bone-implant interfaces and in-vivo degradation of PHB composites for load-bearing applications’, Journal of the Mechanical Behavior of Biomedical Materials, 53, 104–18
Middleton, J., and A. Tipton (2000), ‘Synthetic biodegradable polymers as orthopedic devices’, Biomaterials, 21, 2335–46
Myung, D., W. Koh, J. Ko, Y. Hu, M. Carrasco and J. Noolandi (2007), ‘Biomimetic strain hardening in interpenetrating polymer network hydrogels’, Polymer, 48 (18), 5376–87
Narayanan, G., V. Vernekar, E. Kuyinu and C. Laurencin (2016), ‘Poly (lactic acid)-based biomaterials for orthopaedic regenerative engineering’, Advanced Drug Delivery Reviews 107, 247–76
O’Brien, F. J. (2011), ‘Biomaterials & scaffolds for tissue engineering’, Materials Today, 14 (3), 88–95
Odelius, K., A. Höglund, S. Kumar, M. Hakkarainen, A. K. Ghosh, N. Bhatnagar and A.-C. Albertsson (2011), ‘Porosity and pore size regulate the degradation product profile of polylactide’, Biomacromolecules, 12 (4), 1250–58
Ogden, K., N. Ordway, D. Diallo, G. Tillapsaugh-Fay and C. Aslan (2014), ‘Dimensional accuracy of 3D printed vertebra’, Medical Imaging 2014: Image-Guided Procedures, Robotic Interventions, and Modeling, 9036, 1–8
Owen, A. J., J. Z. Skrbic and V. Divjakovic (1992), ‘Crystallization and melting behaviour of PHB and PHB / HV copolymer’, Polymer, 33 (7), 1563–67
Pamula, E. and E. Dryzek (2008), ‘Structural changes in surface-modified polymers for medical applications’, Acta Physica Polonica, A113 (5), 1485–93
Pappuru, S., and D. Chakraborty (2019), ‘Progress in metal-free cooperative catalysis for the ring-opening copolymerization of cyclic anhydrides and epoxides’, European Polymer Journal, 121, 1–12
Pena, E. S., E. G. Graham-Gurysh, E. M. Bachelder and K. M. Ainslie (2021), ‘Design of biopolymer-based interstitial therapies for the treatment of glioblastoma’, International Journal of Molecular Sciences, 22 (23), 13160
Pereira, H., I. Cengiz, F. Silva, R. Reis and J. Oliveira (2020), ‘Scaffolds and coatings for bone regeneration’, Journal of Materials Science: Materials in Medicine, 31 (27), 1–16
Poulopoulou, N., D. Smyrnioti, G. Nikolaidis, I. Tsitsimaka, E. Christodoulou and D. Bikiaris (2020), ‘Sustainable plastics from biomass: Blends of polyesters based on 2,5-furandicarboxylic acid’, Polymers, 12 (225), 1–21
Prasad, A. (2021), ‘State of art review on bioabsorbable polymeric scaffolds for bone tissue engineering’, Materials Today: Proceedings, 44, 1391–1400
Reddy, M., D. Ponnamma, R. Choudhary, K. Sadasivuni (2021), ‘A comparative review of natural and synthetic biopolymer composite scaffolds’, Polymers, 13 (1105), 1–51
Revati, R., M. Majid and M. Normahira (2015), ‘Biodegradable poly (lactic acid) scaffold for tissue engineering: A brief review’, Journal of Polymer Science and Technology, 1 (1), 16–24
Rezwan, K., Q. Chen, J. Blaker and A. Boccaccini (2006), ‘Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering’, Biomaterials, 27 (18), 3413–31
Rizzi, R., C. Bearzi, A. Mauretti, S. Bernardini, S. Cannata and C. Gargioli (2012), ‘Tissue engineering for skeletal muscle regeneration’, Muscles Ligaments Tendons Journal, 2 (3), 230–34
Robert, J. and K. Aubrecht (2008), ‘Ring-opening polymerization of lactide to form a biodegradable polymer’, Journal of Chemical Education, 85 (2), 1–3
Roy, T., J. Simon, J. Ricci, E. Rekow, V. Thompson, V. and J. Parsons, J. (2003), ‘Performance of degradable composite bone repair products made via three-dimensional fabrication techniques’, Journal of Biomedical Materials Research Part A, 66 (2), 283–91
Sensini, A., G. Massafra, C. Gotti, A. Zucchelli and L. Cristofolini (2021), ‘Tissue engineering for the insertions of tendons and ligaments: an overview of electrospun biomaterials and structures’, Frontiers in Bioengineering and Biotechnology, 9, 1–23
Shebi, A. and S. Lisa (2018), ‘Pectin mediated synthesis of nano hydroxyapatite-decorated poly (lactic acid) honeycomb membranes for tissue engineering’, Carbohydrate Polymers, 201, 39–47
Shi, J., L. Yu and J. Ding (2021), ‘PEG-based thermosensitive and biodegradable hydrogels’, Acta Biomaterialia, 128, 42–59
Shick, T., A. Kadir, N. Ngadiman and A. Ma’aram (2019), ‘A review of biomaterials scaffold fabrication in additive manufacturing for tissue engineering’, Journal of Bioactive and Compatible Polymers, 34 (6), 415–35
Su, N., J. Yang, Y. Xie, X. Du, H. Chen and H. Zhou (2019), ‘Bone function, dysfunction and its role in diseases including critical illness’, International Journal of Biological Sciences, 15 (4), 776–87
Tan, L., W. Zhu and K. Zhou (2020), ‘Recent progress on polymer materials for additive manufacturing’, Advanced Functional Materials, 30, 1–54
Temple, J., D. Hutton, B. Hung, P. Huri, C. Cook and R. Kondragunta (2014), ‘Engineering anatomically shaped vascularized bone grafts with hASCs and 3D-printed PCL scaffolds’, Journal of Biomedical Materials Research Part A, 102 (12), 4317–25
Teoh, S., B. Goh and J. Lim (2019), ‘Three-dimensional printed polycaprolactone scaffolds for bone regeneration success and future perspective’, Tissue Engineering Part A, 25 (13–14), 931–35
Tschan, M., R. Gauvin and C. Thomas (2021), ‘Controlling polymer stereochemistry in ringopening polymerization: A decade of advances shaping the future of biodegradable polyesters’, Chemical Society Review, 50, 13587–608
Turco, R., G. Santagata, I. Corrado, C. Pezzella and M. Di Serio (2020), ‘In vivo and post-synthesis strategies to enhance the properties of PHB-based materials: A review’, Frontiers in Bioengineering and Biotechnology, 8, 1–31
Van Wouwe, P., M. Dusselier, E. Vanleeuw and B. Sels (2016), ‘Lactide synthesis and chirality control for polylactic acid production’, ChemSusChem, 9 (9), 907–21
Verhoeven, J., R. Schaeffer, J. Bouwstra and H. Junginger (1989), ‘The physico-chemical characterization of poly (2-hydroxyethylmethacrylate-co-methacrylic acid: 2. Effect of water, PEG 400 and PEG 6000 on the glass transition temperature’, Polymer, 30 (10), 1946–50
Wanamaker, C. L., W. B. Tolman and M. A. Hillmyer (2009), ‘Hydrolytic degradation behavior of a renewable thermoplastic elastomer’, Biomacromolecules, 10 (2), 443–48
Wang, Y. and M. Rudnicki (2012), ‘Satellite cells, the engines of muscle repair’, Nature Reviews Molecular Cell Biology, 13 (2), 127–33
Wei, C., L. Cai, B. Sonawane, S. Wang and J. Dong (2012), ‘High-precision flexible fabrication of tissue engineering scaffolds using distinct polymers’, Biofabrication, 4 (2), 1–12
Williams, D. (2019), ‘Challenges with the development of biomaterials for sustainable tissue engineering’, Frontiers in Bioengineering and Biotechnology, 7 (127), 1–10
Winnacker, M. (2019), ‘Polyhydroxyalkanoates: Recent advances in their synthesis and applications’, European Journal of Lipid Science and Technology, 121, 1–9
Wu, Y., S. Shaw, H. Lin, T. Lee and C. Yang (2006), ‘Bone tissue engineering evaluation based on rat calvaria stromal cells cultured on modified PLGA scaffolds’, Biomaterials, 27 (6), 896–904
Wuisman, P. and T. Smit (2006), ‘Bioresorbable polymers: Heading for a new generation of spinal cages’, European Spine Journal, 15 (2), 133–48
Xiong, Z., W. Cui, T. Sun, Y. Teng, Y. Qu, L. Yang, J. Zhou, K. Chen, S. Yao and X. Guo (2020), ‘Sustained delivery of PlGF-2123-144*-fused BMP2-related peptide P28 from small intestinal submucosa/polylactic acid scaffold material for bone tissue regeneration’, RSC Advances, 10 (12), 7289–300
Xu, M., C. Guo, H. Dou, Y. Zuo, Y. Sun, J. Zhang and W. Li (2019), ‘Tailoring degradation and mechanical properties of poly (ε-caprolactone) incorporating functional ε-caprolactone-based copolymers’, Polymer Chemistry, 10
Yamane, K., H. Sato, Y. Ichikawa, K. Sunagawa and Y. Shigaki (2014), ‘Development of an industrial production technology for high-molecular-weight polyglycolic acid’, Polymer Journal, 46 (11), 769–75
Yang, S., K. Leong, Z. Du and C. Chua (2001), ‘The design of scaffolds for use in tissue engineering. Part I. Traditional factors’, Tissue Engineering, 7 (6), 679–89
Yang, X., Y. Li, X. Liu, Q. Huang, R. Zhang and Q. Feng (2018), ‘Incorporation of silica nanoparticles to PLGA electrospun fibers for osteogenic differentiation of human osteoblast-like cells’, Regenerative Biomaterials, 5 (4), 229–38
Zeng, J.H., S. W. Liu, L. Xiong, P. Qiu, L.H. Ding, S. L. Xiong, J. T. Li, X. G. Liao and Z. M. Tang (2018), ‘Scaffolds for the repair of bone defects in clinical studies: A systematic review’, Journal of Orthopaedic Surgery and Research, 13 (33), 1–14
Zhang, S., L. Chen, Y. Jiang, Y. Cai, G. Xu and T. Tong (2013), ‘Bi-layer collagen/microporous electrospun nanofiber scaffold improves the osteochondral regeneration’, Acta Biomaterialia, 9 (7), 7236–47
Zhang, Y., C. Wang, L. Fu, S. Ye, M. Wang and Y, Zhou (2019), ‘Fabrication and application of novel porous scaffold in situ-loaded graphene oxide and osteogenic peptide by cryogenic 3d printing for repairing critical-sized bone defect’, Molecules, 24 (9), 1–20
Zhang, B., L. Wang, P. Song, X. Pei, H. Sun, L. Wu, C. Zhou, K. Wang, Y. Fan and X. Zhang (2021), ‘3D printed bone tissue regenerative PLA/HA scaffolds with comprehensive performance optimizations’, Materials & Design, 201, 109490
Zhao, H. (2019), ‘Chapter One – Enzymatic polymerisation to polyesters in nonaqueous solvents’, Methods in Enzymology, 627, 1–21
Zimmer Biomet (2019), ‘Lactosorb® resorbable fixation system’, available at https://www.zimmerbiomet.com/medical-professionals/cmf/lactosorb-resorbable-fixation-system.html, accessed 15 November 2020
Aliphatic: Relating to or denoting organic compounds in which carbon atoms form open chains (as in the alkanes), not aromatic rings.
Biocompatibility: The property of a material being compatible with living tissue by not producing toxic or immunological responses.
Biomimetic: Emulating natural structures or systems.
Composite: A material which is produced from the combination of two or more constituent materials resulting in properties which are superior to those of the original components for a specific application.
Copolymer: A polymer fabricated from more than one monomer species.
Degradable: Capable of being slowly broken down into simple parts.
Esterification: Chemical reaction where two reactants form an ester as the reaction product.
Hydrogel: A cross-linked hydrophilic polymeric gel in which the liquid component is water.
Kinetics: The rates of chemical or biochemical reactions.
Polymer: A substance which has a molecular structure built up chiefly or completely from a large number of similar units bonded together.
Scaffold: Materials that have been engineered to cause desirable cellular interactions to contribute to the formation of new functional tissues for medical purposes.
Synthetic: A substance made by chemical synthesis.
Tissue engineering: A field that blends engineering and biology to develop a means by which biological functionality can be restored, maintained or improved following major accidents, surgery or clinical treatments.
To cite this paper please use the following details: Hartley, E., Moon, H. and Neves, A.I.S. (2022), 'Biodegradable Synthetic Polymers for Tissue Engineering: A Mini-review', Reinvention: an International Journal of Undergraduate Research, Volume 15, Issue 1, https://reinventionjournal.org/article/view/801. Date accessed [insert date]. If you cite this article or use it in any teaching or other related activities please let us know by e-mailing us at Reinventionjournal@warwick.ac.uk.