Adam Paul Plotkin, Cornell College
The anorexia of ageing, a reduction in food intake with increased age, is associated with negative health outcomes such as sarcopenia frailty, cachexia morbidity and mortality. Pharmacological agents such as appetite stimulants have been a major focus to combat the anorexia of ageing; however, these medications are linked to various adverse side effects. Therefore, understanding the physiological causes of reduced appetite may lead to the creation of innovative intervention strategies in the ageing population. Current research has identified the pro-opiomelanocortin (POMC) and Agouti-related peptide (AgRP) neuronal subsets of the arcuate nucleus (ARC) as the centre of appetite regulation. This review investigates the current understanding of appetite regulation and subsequent dysregulation with age, and the age-associated changes in the anorectic (appetite-suppression) and orexigenic (appetite-stimulating) pathways, thereby implicating the POMC and AgRP neurons. It primarily investigates the physiological changes underlying appetite reduction with ageing to orient future interventions to combat the anorexia of ageing.
Keywords: Anorexia of ageing, appetite dysregulation with age, appetite regulation, biology of ageing
The dysregulation of appetite control is common during the natural ageing process, resulting in a reduction of food intake. This process is often called ‘the anorexia of ageing’ and is associated with sarcopenia, frailty, cachexia, morbidity and mortality (Simmons et al., 2008). In the elderly population (defined as those 65+ years old), the anorexia of ageing impacts approximately 25 per cent of home-dwellers, 62 per cent in hospital settings and 82 per cent in nursing home populations (Roy et al., 2016).
Several appetite stimulants have been tested for efficacy as a pharmacological intervention for combatting appetite reduction in older adults. The main stimulant studied in the malnourished elderly population since the early 2000s, megestrol acetate (Megace), has demonstrated mixed results in a few small and randomised trials (Persons and Nicholls, 2007). For instance, one study randomly assigned nursing home residents to receive placebo or Megace (800mg per day) for 12 weeks, followed by 13 weeks off treatment. There were no significant differences in weight gain and body composition between treatment groups at 12 weeks. However, improvements in appetite, quality of life and wellbeing were significantly greater in Megace-treated residents based on participant feedback. Last, at 25 weeks post-treatment initiation, 61.9 per cent of Megace-treated residents had gained greater than or equal to 1.82kg compared to 21.7 per cent of placebo residents (Yeh et al., 2001). However, a later study reported no significant improvement in weight, functional status or health-related quality of life following a 63-day treatment period of varying doses of Megace (Reuben et al., 2005). The three-week difference with regards to Megace administration between these two studies could explain the discrepancy in the results. Therefore, further clinical trials are necessary to discern the proper Megace dosing regimen in this population.
In addition, the administration of Megace has been linked to adverse side effects such as diarrhoea, cardiomyopathy, leukopenia, depression and pulmonary embolism (Gurvich and Cunningham, 2000; PDR Search, 2020). Due to the lack of weight-gain success in limited clinical trials, and several adverse side effects, Beers Criteria (standards for medication use for those over the age of 65; American Geriatrics Society, 2019) has labelled Megace as a potentially inappropriate medication to treat cachexia/poor appetite (AGS, 2022). These findings necessitate further comprehension of the biochemical and physiological basis for appetite regulation to implement novel intervention strategies (Table 1).
| Change with age | Impact on pathways | Interventions | Example of major ongoing research questions |
| Decreased sense of taste | Blunts hedonistic pathway, favouring anorectic pathway | Sorbet Increases Salivation | Although successful in two small clinical trials, can this intervention elicit increased food intake on a larger scale and over a longer period of time? |
| Decreased sense of smell | Blunts hedonistic pathway, favouring anorectic pathway | Supplementation with zinc sulphates, Vitamin A, and Vitamin B3 + the removal of sucralose | What is the relationship between common drugs/supplements taken by older adults and the pathophysiology of presbyosmia? |
| Decreased gastric motility | Blunts orexigenic pathway | Reduce dietary fat intake, anti-obesity medication (Orlistat), daily bouts of physical activity | What are the health implications of reducing/removing dietary fat from the diet of older adults? How safe is taking Orlistat over a continued period of time? What type of exercise, and undertaken for how long, demonstrates the greatest improvement in gastric motility in older adults? |
| Decreased NPY levels | Blunts orexigenic pathway | More structured mealtimes (similar time each day) | Several studies in non-human models demonstrate increases in NPY levels, but does the same occur in humans? |
| Decreased ORX and MCH levels | Blunts orexigenic pathway and favours anorectic pathway | Spread-out mealtimes | More spaced-out mealtimes allow more time for digestion, but what is the physiological impact on ORX and MCH levels? |
| Decreased AgRP levels | Blunts orexigenic pathway and favours anorectic pathway | Administration of glucocorticoids and/or CPT-1 inhibitors | What is the proper dosage of each medication, and are there any adverse effects in prolonged usage? |
| Hypoxia-induced weight loss potentially linked to ischaemic stroke | Blunts orexigenic pathway and favours anorectic pathway | Typical treatments for ischaemic stroke (aspirin, beta blockers, nitrates, surgery, etc.) | How do hypoxic conditions impact AgRP levels? Do typical ischaemic treatments help restore baseline levels of appetite-regulating metabolites? |
| Decreased ghrelin levels | Blunts orexigenic pathway | Ghrelin mimetics and liquified meals | How safe is ghrelin-agonist administration in older adults? How compliant are older adults with regards to maintaining a primarily liquid diet? |
| Increased leptin levels | Blunts orexigenic pathway and favours anorectic pathway | Avoid inflammatory foods, physical activity, proper sleep, alpha lipoic acid, fish oil | Are non-pharmaceutical approaches to decrease leptin levels effective, or should pharmaceutical approaches be pursued? |
To construct this narrative review, the author searched PubMed using the keywords ‘POMC and AgRP’ and the filter ‘review’. No time limits were used. One hundred and thirty-seven articles were found (September 2020). Several of these papers, plus additional publications found in the references, were used to construct a framework of POMC and AgRP action in the ARC and their downstream effects.
For a better understanding of the physiology of ageing and the pathophysiology of the anorexia of ageing, the phrase ‘anorexia of ageing’ was searched in PubMed. There was no time limit set. Inclusion criteria included ‘clinical trial’ and participants aged 65+. Twenty-four articles were found (February 2021). Additional clinical trials cited in the references of these studies were used to complete the narrative review. Clinical trials investigating pathological and social factors/interventions were excluded, as were studies regarding anorexia nervosa in elderly populations.
Appetite is a part of the energetic equilibrium required for weight regulation. Today, it is evident that appetite is part of a complex process in which a signalling pathway occurs between the digestive system, the endocrine system, the brain and sensory nerves to modulate hunger, satiation and satiety. However, this complex process can be more simplistically thought of as a system composed of two complementary pathways: the hedonistic pathway and the homeostatic pathway (Andermann and Lowell, 2017). The homeostatic pathway is driven by internal and metabolic signals (e.g. hormones) to maintain energy balance. In contrast, the hedonistic pathway is driven by environmental signals (such as food presentation) based on reward and desire to consume palatable food for pleasure (Berthoud, 2011; Lee and Dixon, 2017). The intricate nature of appetite regulation depends on the balance of the hedonistic and homeostatic pathways (Lee and Dixon, 2017). This review will primarily focus on the homeostatic pathway.
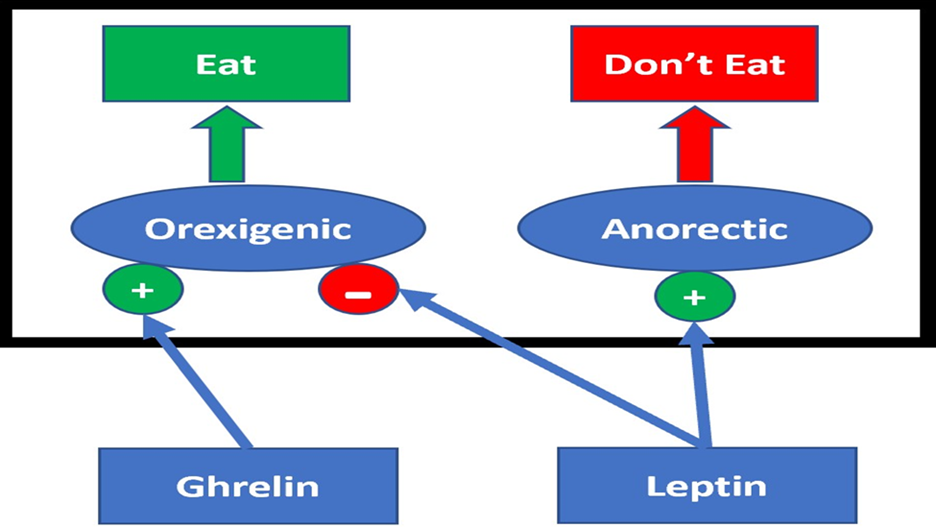
Homeostatic appetite is divided into two general pathways. These pathways are thought of as the anorectic pathway, which functions by suppressing appetite, and the orexigenic pathway, which functions by ‘de-suppressing’ appetite. These pathways are centralised in the ARC of the hypothalamus and project to similar regions of the brain, such as the lateral hypothalamus and paraventricular nucleus (Cui et al., 2017).
Within the ARC exists two distinct subsets of neurons implicated in energy balance. The first subset – referred to as AgRPARC neurons – express AgRP, neuropeptide Y (NPY), and GABA. This subset of neurons in the ARC modulates the orexigenic pathway and predominately functions through inhibiting the anorectic pathway, thereby ‘de-suppressing’ appetite (Paeger et al., 2017). The second subset of neurons in the ARC, referred to as POMCARC neurons, express the peptide neurotransmitter POMC and cocaine-amphetamine-regulated transcript (CART). This subset of neurons in the ARC modulates the anorectic pathway and functions primarily by activating neuronal networks that suppress feeding (Cui et al., 2017). The subsequent activation and inhibition of these distinct ARC neuronal populations are controlled by physiological signals that fluctuate with the body’s energy levels. The peptide hormone leptin is the main controller of the anorectic pathway, whereas ghrelin is the main controller of the orexigenic pathway (Cui et al., 2017; Figure 1).

Research demonstrates that the physiology of hedonistic and homeostatic appetite control changes with age, which may contribute to the onset of the anorexia of ageing (Atalayer and Astbury, 2013). A better understanding of the biochemical and physiological changes in appetite regulation with age may potentially lead to the creation of new intervention strategies to combat the anorexia of ageing and its negative health outcomes.
For instance, alterations in chemosensory detection of food play a role in the suppression of appetite in the elderly population, thereby resulting in decreased food intake (Hays and Roberts, 2006). For example, a moderate loss of taste occurs during the normal course of ageing in a healthy adult (Schiffman, 1997). This is demonstrated in a recent cross-sectional analysis of 359 community-dwelling Dutch senior citizens (age 65–93), which established that 9.2 per cent of the sample had poor taste, and self-reported poor taste was associated with poor appetite (Fluitman et al., 2021).
In response to this loss of taste with age, a novel pilot test referred to as ‘Sorbet Increase Salivation’ (SIS) was conducted in the last decade to combat xerostomia (dry mouth) and ultimately increase food intake. This study demonstrated that elderly subjects who consumed two ounces of lemon-lime sorbet prior to lunch/dinner ate a more significant amount of food and had significant increases in salivation when compared to those who consumed a non-citrus drink prior to lunch/dinner. On average, residents consumed 208 ± 98 grams of food pre-sorbet compared to 253 ± 96 grams of food post-sorbet (Crogan et al., 2014). Hence, the SIS approach offers a novel method to increase food intake via salivation stimulation while offering the potential to entice older individuals to eat more. However, this study included a small sample size (n=22), and demonstrates a need for further studies with larger sample sizes.
In addition, the sense of smell also deteriorates with the progression of ageing (presbyosmia), as older individuals demonstrate a higher odour detection and recognition threshold (the minimal concentration of an aroma necessary to be detected by the human nose; Doty, 1991). From 2011 to 2014, the National Health and Nutrition Examination Survey included a ‘Chemosensory’ component in which it was determined that about 12 per cent of individuals aged 40 or older experience alterations in their sense of smell, and this value increases to roughly 40 per cent in those aged 80 or older (NHANES, 2011–2012; NHANES, 2013–2014). Thus, a deterioration in smell as one ages may dampen the hedonistic pathway of appetite.
Various avenues have been tested to combat presbyosmia, such as the addition of zinc salts and vitamins A and B3 to the diet, as well as the removal of the organochlorine sweetener sucralose. However, these approaches have been met with varying levels of success, most likely due to the unique biological effect of different drug types. Therefore, further research is needed to understand the pathophysiology underlying presbyosmia to formulate more targeted approaches to restore one’s sense of smell (Schiffman, 1983; Schiffman, 1997; Schiffman, 2007; Schiffman and Rother, 2013; Schiffman and Zervakis, 2002).
Modifications in the gastrointestinal tract also play a role in decreased food intake in the elderly population (Hays and Roberts, 2006). Elderly individuals require a longer period to digest the same nutrients when compared to younger individuals, in which the extra distension placed on the antral stomach (the portion of stomach that holds broken-down nutrients) is directly related to lower sensations of satiation (Clarkston et al., 1997; Moriguti et al., 2000; Rolls et al., 1995; Soenen et al., 2015). Sturm et al. (2004) showed that after pre-loading with a nutrient-rich liquid, the antral area of older individuals was greater than that of younger subjects who consumed the same nutrient-rich liquid. This extra distention placed on the antral stomach creates a longer digestion period, as demonstrated by both a slower gastric emptying time (time it takes for 50 per cent gastric emptying) and post-prandial hunger being inversely associated with gastric emptying in elderly individuals (Clarkston et al., 1997).
One novel approach to improve gastric emptying in older adults has been the use of the obesity management drug, Orlistat (a pancreatic lipase inhibitor; Drug Bank, 2021). However, to date, there has only been one clinical trial using Orlistat specifically in older adults. This study, in which Orlistat administration inhibited the usual decrease in gastric emptying following dietary fat consumption, demonstrated promise. Yet only nine older adults were studied (Tai et al., 2011), requiring further study to determine efficacy and safety of this drug.
Therefore, a different approach, such as the participation in regular bouts of light to moderate physical activity prior to mealtime, may be of benefit to this population (Bi and Triadafilopoulos, 2003). However, more research is needed to determine the duration and type of physical activity that not only helps improve gastric emptying in older individuals but also is deemed safe and enjoyable.
Orexigenic appetite is regulated by the peptide hormone ghrelin (Cui et al., 2017). Ghrelin is synthesised by endocrine cells located in the gut and is the only known orexigenic gut peptide. Ghrelin concentrations increase pre-prandially in humans and during bouts of food deprivation in animals (Cui et al., 2017; Mani et al., 2019; Uchida et al., 2020). Ghrelin can access the ARC through an incomplete blood–brain barrier adjacent to the median eminence (Cabral et al., 2017; Uriarte et al., 2018). Inside the ARC, ghrelin’s main targets are AgRPARC neurons (Méquinion et al., 2020), where it binds the growth hormone secretagogue receptor (Cui et al., 2017).
Activated AgRPARC neurons directly innervate the lateral hypothalamus and release NPY neuropeptides into this brain region (Wasinski et al., 2020). NPY neuropeptides bind to a Class-A G-protein coupled receptor expressed by orexin neurons found within the lateral hypothalamus (ORXLHA), triggering the release of an orexigenic neuropeptide, orexin (Gene Group, 2020; Guo et al., 2018; Okumura and Nozu, 2011). ORX neuropeptides subsequently diffuse to various regions of the central nervous system, where they bind to ORX receptors and initiate further orexigenic actions (O’Leary, 2014).
ORX neuropeptides stimulate vagal and spinal nerves (as well as the enteric plexus), mucous and musculature of the gut (Guo et al., 2018). These areas impact gut motility (Ahima and Antwi, 2008; Baccari, 2010; Bulbul et al., 2010). ORX peptides increase enteric motor neuron activity and smooth muscle contractility within the duodenum, resulting in enhanced gastric emptying (Squecco, et al., 2011; Figure 2). The enhanced rate of gastric emptying via enteric ORX peptide excitation thereby stimulates the orexigenic pathway.
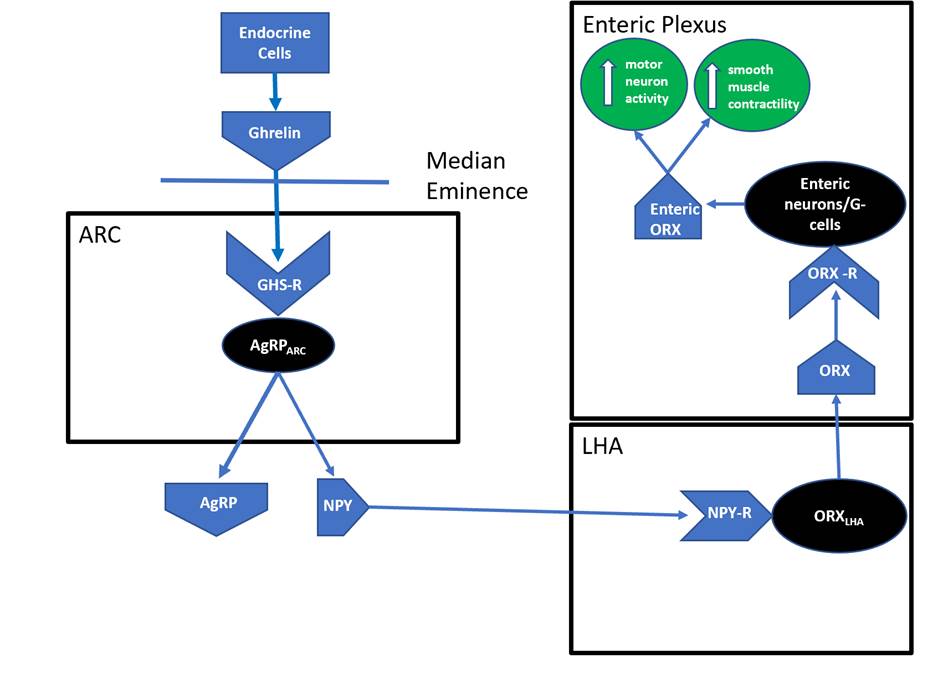
The orexigenic effects initiated by activated ORXLHA neurons extend beyond enhancing gastric motility. Within the lateral hypothalamus, ORX neuropeptides bind to ORX receptors expressed on melanin-concentrating hormone neurons (MCHLHA). This stimulates the release of MCH neuropeptides, an antagonist of α-MSH, a key anorectic peptide hormone (see ‘Anorectic pathway changes with age’, below), thereby blunting the anorectic pathway (Barson et al., 2013; Diniz et al., 2019; Madelaine et al., 2020).
However, changes in the gene expression of key orexigenic proteins throughout the ageing process result in the suppression of appetite in elderly individuals (Wernette et al., 2011). For example, the gene expression and resulting protein content of NPY and its receptors decreases with age in rodents (Morley, 2001; Takeda et al., 2010), resulting in a reduction of ORXLHA neuronal stimulation and the consequential blunting of the orexigenic pathway.
In addition, reduction in the gene expression of the orexigenic peptide hormones ORX and MCH, and a decrease in the prevalence of ORX receptors located throughout areas of the hypothalamus, are associated with the normal ageing process (Kappeler et al., 2003; Porkka-Heiskanen et al., 2004; Wernette et al., 2011). This results in less stimulation of the orexigenic pathway and a decreased ability to inhibit the anorectic pathway.
To combat these changes, one may alter the meal structure of elderly individuals to include more spread-out feedings, as this would allow more time for digestion and improve gastric emptying rate (Jackson et al., 2007). Additionally, the introduction of a day-to-day meal plan may result in increased levels of NPY. Animals on a trained eating regimen demonstrate increased NPY levels prior to the normal mealtime (Campos et al., 2012; Chen et al., 2019; Kalra et al., 1991; Yoshihara et al., 1996). However, clinical trials are necessary to determine the impact of meal timing on NPY levels in humans.
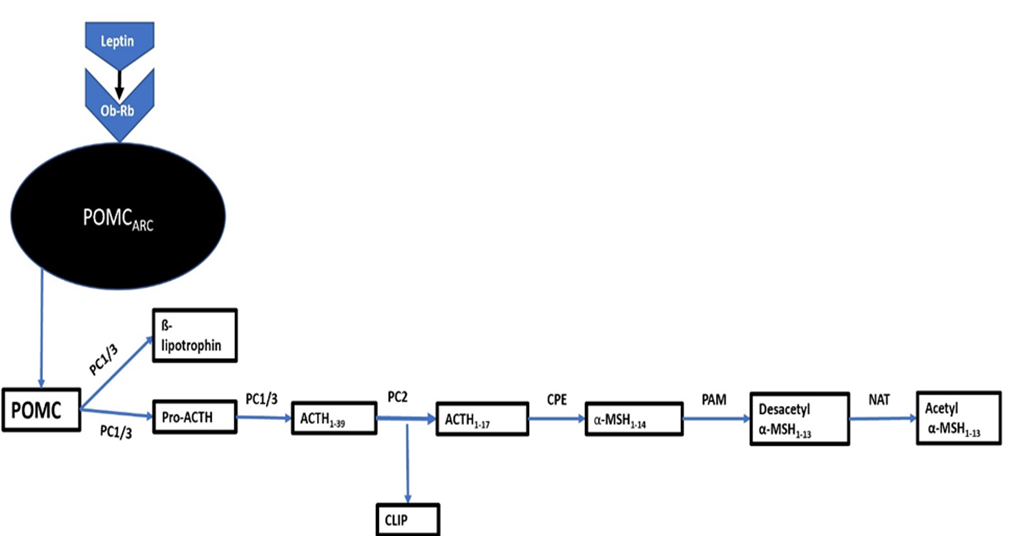
Leptin is primarily secreted by white adipocytes, which are fat storage cells located in the subcutaneous layer of skin and between muscles and internal organs (Harris, 2014). Leptin accesses the ARC through the median eminence (Cui et al., 2017; Scott et al., 2009), and its main targets are POMCARC neurons (Dodd et al., 2015). There are at least six leptin receptor isoforms, the longest of which, Ob-Rb, is expressed on POMCARC neurons (Harris, 2014; Wauman et al., 2017). Leptin binds Ob-Rb located on POMCARC neurons and activates the anorectic pathway (Dodd et al., 2015).
Leptin-activated POMCARC neurons induce synthesis of the polypeptide hormone precursor, POMC (Cui et al., 2017; Morton et al., 2006). Activated POMC peptide hormones are processed by several enzymes to form the small, biologically active peptide acetyl-α-melanocyte stimulating hormone (α-MSH; D’Agostino and Diano, 2010; Morton et al., 2006; Figure 3).
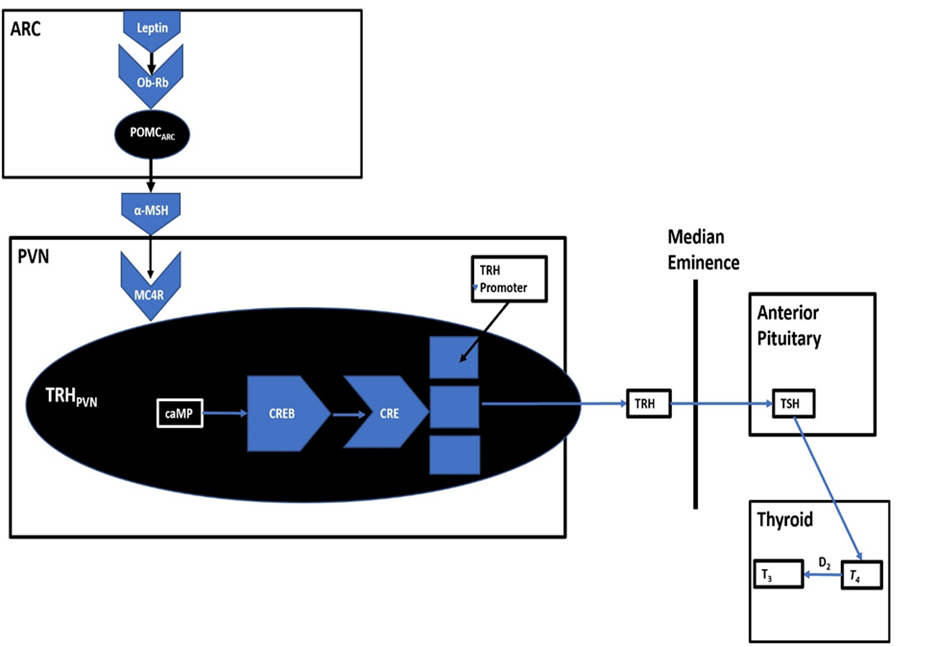
POMCARC neurons directly innervate the paraventricular nucleus (Bell et al., 2018; Cui et al., 2017; Morton et al., 2006). Following activation, α-MSH enters the paraventricular nucleus and binds melanocortin receptors (MCR). α-MSH is an agonist for the MCR system constituents, which are imperative for the continuation of the anorectic pathway (Bell et al., 2018; Cui et al., 2017; Morton et al., 2006).
Within the paraventricular nucleus resides thyrotropin-releasing hormone neurons (TRHPVN), which express MCRs (Decherf et al., 2010). The activation of MCRs expressed on TRHPVN neurons by α-MSH results in a signalling cascade culminating in the production of triiodothyronine (T3), a regulator of the body’s metabolic rate (Amin et al., 2011; Campos et al., 2020; Figure 4). T3 administration upregulates mitochondrial uncoupling proteins (UCPs), resulting in increased internal thermogenesis (Barbe et al., 2001; Bézaire et al., 2007; Lanni et al., 1999; Mullur et al., 2014; Puigserver, 2005; Silva, 2011; Weitzel et al., 2001). The increase in internal thermogenesis has been proposed to induce appetite suppression (Coppola et al., 2004; Harris et al., 2001; Josic et al., 2010; Kong et al., 2004; Perello et al., 2006, Strominger and Brobeck, 1953).
Like the changes in gene expression in orexigenic signalling proteins, the ageing process has been linked to changes in anorectic signalling, thus amplifying appetite suppression as one ages (Wernette et al., 2011). For instance, the ageing process results in reduced AgRP gene expression in rodents (Kmiec, 2006; Zhang et al., 2004). The reduction in AgRP gene expression ‘de-suppresses’ the anorectic pathway. AgRP is also an endogenous antagonist for α-MSH (Zhang et al., 2017). Therefore, stimulated AgRPARC neurons in elderly individuals are not able to suppress α-MSH as effectively as a younger individual, resulting in an inability to suppress the anorectic pathway. Several avenues to increase AgRP gene expression have been demonstrated in rodent models, such as the administration of glucocorticoids (Shimizu et al., 2008) and a reduction in long-chain acyl-CoA in the ARC via carnitine palmitoyltransferase-1 inhibition (CPT-1; Obici et al., 2003). However, further research is needed not only to determine proper dosing but also to troubleshoot potential adverse effects of these pharmaceutical approaches.
Finally, research demonstrates that exposure to hypoxia (oxygen deprivation at the level of the tissue) stimulates weight loss (Kayser and Verges, 2013; Netzer et al., 2008; Quintero et al., 2010), most likely due to reduced appetite and subsequent decreased food intake (Benso et al., 2007; Kalson et al., 2010; Westerterp and Kayser, 2006). Interestingly, in hypoxic conditions, ghrelin levels decrease (Matu et al., 2017; Wasse et al., 2012). At the same time, both leptin (Lippl et al., 2010; Mekjavic et al., 2016; Shukla et al., 2005; Snyder et al., 2008) and POMC (Varela et al., 2017; Zhang et al., 2011) levels increase, and the effect on AgRP remains unknown (Kietzmann and Mäkelä, 2021). This is an intriguing connection, because roughly 800,000 strokes occur annually in the USA, and approximately 87 per cent of those are considered ischaemic (inadequate blood supply to an organ or part of the body). Additionally, approximately three-quarters of all strokes occur in individuals aged 65 years or older (Benjamin et al., 2018). Thus, it appears that there could be a relationship between hypoxia-induced pathophysiology and the reduction and increase of orexigenic and anorectic metabolites, respectively. However, new research initiatives are needed to further elucidate this relationship and discover whether typical ischaemic treatments such as medication, surgery and lifestyle alterations also impact these metabolites (Mayo Clinic, 2021).
Plasma ghrelin levels decrease during the normal ageing process (Hays and Roberts, 2006). Rigamonti et al. (2002) showed that normal-weight older subjects exhibited 35 per cent lower ghrelin plasma concentrations when compared to normal-weight younger subjects. Additionally, in younger subjects, post-prandial ghrelin levels returned to fasting levels approximately two to four hours after meal completion, while elderly subjects did not exhibit return-to-fasting level ghrelin concentrations in a post-prandial setting (Di Francesco et al., 2008).
In contrast to plasma ghrelin decreasing with age, plasma leptin concentrations increase with age (Atalayer and Astbury, 2013). This is evident by both pre- and post-prandial leptin levels in elderly individuals being higher than the leptin levels present in younger individuals (Di Francesco et al., 2006; Zamboni et al., 2004).
Therefore, rising leptin levels and falling ghrelin levels are potential targets to combat the anorexia of ageing. Accordingly, avoiding inflammatory foods (e.g. trans fats, refined sugars), consuming anti-inflammatory foods (e.g. fatty fish), participating in moderate physical activity, and supplementing with alpha lipoic acid and fish oil may all aid in leptin stabilisation (Abd El-Kader et al., 2013; Ellulu et al., 2016; Huerta et al., 2015; Reseland et al., 2001; Shapiro et al., 2008; Spiegel et al., 2004). Also, in the past decade, steps have been taken to develop ghrelin mimetics, such as macimorelin for the diagnosis of growth hormone deficiency, anamorelin for the treatment of cancer cachexia, and relamorelin for the treatment of gastrointestinal disorders (Currow and Abernathy, 2014; Koch, 2013; Van der Ploeg et al., 2014). Furthermore, liquified meals offer promise of increased appetite in older adults. Elderly individuals who consume liquified meals exhibit higher post-prandial ghrelin composites when compared to those consuming isoenergetic solid meals (Tieken et al., 2007). Nevertheless, despite the potential for liquid meals to increase appetite in older individuals, longer intervention trials are needed to determine compliance to a predominately liquid-based diet in this population.
Appetite incorporates both hedonistic and homeostatic mechanisms, which are centred through the ARC and stem out to various regions of the body. Ghrelin and AgRPARC neurons control the orexigenic pathway in which activation stimulates hunger. Leptin and POMCARC neurons, on the other hand, control the anorectic pathway in which activation primarily suppresses hunger. Comprehension of appetite regulation and the subsequent biochemical and physiological modifications that occur during ageing allows for the implementation of intervention strategies to combat the anorexia of ageing. The AgRPARC and POMCARC neuronal subsets are prime targets for such interventions.
Funding
No Funding from external organisations was obtained
Conflict of Interest
The author declares no conflict of interest.
Figure 1: Basic overview of appetite regulation.
Figure 2: ORXLHA impacts gastric motility.
Figure 3: α-MSH synthesis.
Figure 4: ARC control of TRH levels.
Table 1: Approaches to combat the anorexia of ageing.
Abd El-Kader, S., A. Gari and A. Salah El-Den (2013), ‘Impact of moderate versus mild aerobic exercise training on inflammatory cytokines in obese type 2 diabetic patients: A randomized clinical trial’, Afr Health Sci. 13 (4) 857–63, available at https://pubmed.ncbi.nlm.nih.gov/24940305/, accessed 18 August 2020
American Geriatrics Society (2019), ‘AGS Beers Criteria for Potentially Inappropriate Medication Use in Older Adults Guideline Summary’, available at https://www.guidelinecentral.com/guideline/340784/, accessed 9 January 2022
Ahima, R. S. and D. A. Antwi (2008), ‘Brain regulation of appetite and satiety’, Endocrinology and Metabolism Clinics of North America, 37 (4), 811–23, available at https://pubmed.ncbi.nlm.nih.gov/19026933/, accessed 30 April 2020
Amin, A., W. S. Dhillo and K. G. Murphy (2011), ‘The central effects of thyroid hormones on appetite’, Journal of Thyroid Research, 1–7, available at https://www.hindawi.com/journals/jtr/2011/306510/, accessed 3 May 2020
Andermann, M. and B. Lowell (2017), ‘Toward a wiring diagram understanding of appetite control’, Neuron, 95 (4), 757–78
Atalayer, D. and N. Astbury (2013), ‘Anorexia of aging and gut hormones’, Aging and Disease, 04 (05), 264–75, available at http://www.aginganddisease.org/EN/10.14336/AD.2013.0400264, accessed 2 July 2020
Baccari, M. (2010), ‘Orexins and gastrointestinal functions’, Curr. Protein Pept. Sci. 11, 148–55
Barbe P, D. Larrouy, C. Boulanger, E. Chevillotte, N. Viguerie, C. Thalamas, M.O. Trastoy, M. Roques, H. Vidaland and D. Langin (2001), ‘Triiodothyronine-mediated up-regulation of UCP2 and UCP3 mRNA expression in human skeletal muscle without coordinated induction of mitochondrial respiratory chain genes’, FASEB J. (1), 13–15, available at https://pubmed.ncbi.nlm.nih.gov/11099489/, accessed 15 February 21
Barson, J., I. Morganstern and S. Leibowitz (2013), ‘Complementary roles of orexin and melanin-concentrating hormone in feeding behavior’, International Journal of Endocrinology, 1–10, available at https://pubmed.ncbi.nlm.nih.gov/23935621/, accessed 12 February 21
Bell, B., S. Harlan, D. Morgan,D. Guo and K. Rahmouni (2018), ‘Differential contribution of POMC and AgRP neurons to the regulation of regional autonomic nerve activity by leptin’, Molecular Metabolism, 8,1–12
Benjamin E, S. Virani, C. Callaway, A. Chamberlain, A. Chang, S. Cheng, S. Chiuve, M. Cushman, F. Delling, R. Deo, S. de Ferranti, J. Ferguson, M. Fornage, C. Gillespie, C. Isasi, M. Jiménez, L. Jordan, S. Judd, D. Lackland, J. Lichtman, L. Lisabeth, S. Liu, C. Longenecker, P. Lutsey, J. Mackey, D. Matchar, K. Matsushita, M. Mussolino, K. Nasir, M. O’Flaherty, L. Palaniappan, A. Pandey, D. Pandey, M. Reeves, M. Ritchey, C. Rodriguez, G. Roth, W. Rosamond, U. Sampson, G. Satou, S. Shah, N. Spartano, D. Tirschwell, C. Tsao, J. Voeks, J. Willey, J. Wilkins, J. Wu, H. Alger, S. Wong andP. Muntner (2018), ‘American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics–2018 update: A report from the American Heart Association’, Circulation. 20;137 (12)
Benso, A., F. Broglio, G. Aimaretti, B. Lucatello, F. Lanfranco, E. Ghigo and S. Grottoli (2007), ‘Endocrine and metabolic responses to extreme altitude and physical exercise in climbers’, European Journal of Endocrinology, 157 (6), 733–40, available at doi:10.1530/EJE-07–0355 (psu.edu), accessed 14 February 21
Berthoud H (2011), ‘Metabolic and hedonic drives in the neural control of appetite: Who is the boss?’, Curr Opin Neurobiol, 21 (6), 888–96
Bézaire, V., E. Seifert and M. Harper (2007), ‘Uncoupling protein-3: Clues in an ongoing mitochondrial mystery’, The FASEB Journal, 21 (2), 312–24, available at https://faseb.onlinelibrary.wiley.com/doi/abs/10.1096/fj.06-6966rev, accessed 6 May 2020
Bi, L. and G. Triadafilopoulos (2003), ‘Exercise and gastrointestinal function and disease: An evidence-based review of risks and benefits’, Clinical Gastroenterology and Hepatology, 1 (5), 345–55
Bulbul, M., R. Babygirija, J. Zheng, K. A. Ludwig and T. Takahashi (2010), ‘Central orexin-A changes the gastrointestinal motor pattern from interdigestive to postprandial in rats’, Auton. Neurosci. 158, 24–30
Cabral, A., M. Cornejo, G. Fernandez, P. De Francesco, G. Garcia-Romero, M. Uriarte, J. Zigman, E. Portiansky, M. Reynaldo and M. Perello (2017), ‘Circulating ghrelin acts on gaba neurons of the area postrema and mediates gastric emptying in male mice’, Endocrinology, 158 (5),1436–49
Campos, V., R. Robaldo, J. Deschamps, F. Seixas, A. McBride, L. Marins, M. Okamoto, L. Sampaio and T. Collares (2012), ‘Neuropeptide Y gene expression around meal time in the Brazilian flounder Paralichthys orbignyanus’, Journal of Biosciences, 37 (2), 227–32
Campos, A., P. Teixeira, F. Wasinski, M. Klein, J. Bittencourt, M. Metzger and J. Donato (2020), ‘Differences between rats and mice in the leptin action on the paraventricular nucleus of the hypothalamus: Implications for the regulation of the hypothalamic pituitary thyroid axis’, Journal of Neuroendocrinology, 32 (9), available at https://pubmed.ncbi.nlm.nih.gov/32840013/, accessed 12 February 21
Chen, Y., R. Essner, S. Kosar, O. Miller, Y. Lin, S. Mesgarzadeh and Z. Knight (2019), ‘Sustained NPY signaling enables AgRP neurons to drive feeding’, eLife, 8, available at https://elifesciences.org/articles/46348, accessed 14 February 21
Clarkston, W., M. Pantano, J. Morley, M. Horowitz, J. Littlefield and F. Burton (1997), ‘Evidence for the anorexia of aging: gastrointestinal transit and hunger in healthy elderly vs. young adults’, American Journal Of Physiology-Regulatory, Integrative And Comparative Physiology, 272 (1), R243–R248, available at https://journals.physiology.org/doi/abs/10.1152/ajpregu.1997.272.1.R243, accessed 22 May 2020
Coppola, J., B. Horwitz, J. Hamilton and R. McDonald (2004), ‘Expression of NPY Y1 and Y5 receptors in the hypothalamic paraventricular nucleus of aged Fischer 344 rats’, American Journal Of Physiology-Regulatory, Integrative And Comparative Physiology, 287 (1), R69–R75, available at https://journals.physiology.org/doi/full/10.1152/ajpregu.00607.2003, accessed 3 May 2020
Crogan, N., A. Simha and C. Morgenstern (2014), ‘Increasing food intake in nursing home residents: Efficacy of the sorbet increases salivation intervention’, Geriatric Nursing, 35 (5), 335–38, available at https://www.sciencedirect.com/science/article/abs/pii/S0197457214001414, accessed 18 August 2020
Cui, H., M. López and K. Rahmouni, K (2017), ‘The cellular and molecular bases of leptin and ghrelin resistance in obesity’, Nat Rev Endocrinol 13, 338–51
Currow D. and A. Abernethy (2014), ‘Anamorelin hydrochloride in the treatment of cancer anorexia-cachexia syndrome’, Future Oncol, 10, 789–802, available at https://pubmed.ncbi.nlm.nih.gov/24472001/, accessed 18 August 2020
Decherf, S., I. Seugnet, S. Kouidhi, A. Lopez-Juarez, M. Clerget-Froidevaux and B. Demeneix (2010), ‘Thyroid hormone exerts negative feedback on hypothalamic type 4 melanocortin receptor expression’, Proceedings Of The National Academy Of Sciences, 107 (9), 4471–76, available at https://www.pnas.org/content/107/9/4471, accessed 3 May 2020
D’Agostino, G. and S. Diano (2010), ‘Alpha-melanocyte stimulating hormone: Production and degradation’, Journal of Molecular Medicine, 88 (12), 1195–1201, available at https://link.springer.com/article/10.1007/s00109-010-0651-0, accessed 2 April 2020
Di Francesco, V., M. Zamboni, E. Zoico, G. Mazzali, A. Dioli, F. Omizzolo, L. Bissoli, F. Fantin, P. Rizzotti, S. Solerte, R. Micciolo and O. Bosello (2006), ‘Unbalanced serum leptin and ghrelin dynamics prolong postprandial satiety and inhibit hunger in healthy elderly: Another reason for the anorexia of aging’, The American Journal Of Clinical Nutrition, 83 (5), 1149–52, available at https://academic.oup.com/ajcn/article/83/5/1149/4649587, accessed 3 July 2020
Di Francesco, V., F. Fantin, L. Residori, L. Bissoli, R. Micciolo A. Zivelonghi, E. Zoico, F. Omizzolo, O. Bosello and M. Zamboni (2008), ‘Effect of age on the dynamics of acylated ghrelin in fasting conditions and in response to a meal’, Journal of the American Geriatrics Society, 56 (7), 1369–70, available at https://onlinelibrary.wiley.com/doi/full/10.1111/j.1532-5415.2008.01732.x, accessed 3 July 2020
Diniz, G., D. Battagello, P. Cherubini, J. Reyes Mendoza, C. Luna Illades, M. Klein, L. Motta-Teixiera, L. Sita, M. Miranda-Anaya, T. Morales and J. Bittencourt (2019), ‘Melanin concentrating hormone peptidergic system: Comparative morphology between muroid species’, Journal Of Comparative Neurology, 527 (18), 2973–3001, available at https://onlinelibrary.wiley.com/doi/abs/10.1002/cne.24723, accessed 3 February 2020
Dodd, G., S. Decherf, K. Loh, S. Simonds, F. Wiede, E. Balland, T. Merry, H. Münzberg, Z. Zhang, B. Kahn, B. Neel, K. Bence, Z. Andrews, M. Cowley and T. Tiganis (2015), ‘Leptin and insulin act on pomc neurons to promote the browning of white fat’, Cell, 160 (1–2), 88–104
Doty, R. (1991), ‘Olfactory capacities in aging and Alzheimer’s disease’, Annals of the New York Academy of Sciences, 640 (1), 20–27, available at https://nyaspubs.onlinelibrary.wiley.com/doi/10.1111/j.1749-6632.1991.tb00185.x, accessed 30 May 2020
Go.drugbank.com (2021), Orlistat | DrugBank Online, available at: https://go.drugbank.com/drugs/DB01083, accessed 13 February 2021
Ellulu M., H. Khaza’ai, I. Patimah, A. Rahmat and Y. Abed (2016), ‘Effect of long chain omega-3 polyunsaturated fatty acids on inflammation and metabolic markers in hypertensive and/or diabetic obese adults: A randomized controlled trial’, Food Nutr Res, 60, available at https://pubmed.ncbi.nlm.nih.gov/26829184/, accessed 18 August 2020
Fluitman, K., A. Hesp, R. Kaihatu, M. Nieuwdorp, B. Keijser, R. IJzerman and M. Visser (2021), ‘Poor taste and smell are associated with poor appetite, macronutrient intake, and dietary quality but not with undernutrition in older adults’, The Journal of Nutrition, 151 (3), 605–14, available at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7948202/, accessed 11 January 2022
Gene Group: CLASS A GPCRs (2020), available at http://flybase.org/reports/FBgg0000041.html, accessed 5 January 2020
Guo, F., S. Gao, L. Xu, X. Sun, N. Zhang, Y. Gong and X. Luan (2018), ‘Arcuate nucleus orexin-a signaling alleviates cisplatin-induced nausea and vomiting through the paraventricular nucleus of the hypothalamus in rats’, Frontiers in Physiology, 9, available at https://www.frontiersin.org/articles/10.3389/fphys.2018.01811/full, accessed 12 February 21
Gurvich T and J. Cunningham (2000). ‘Appropriate use of psychotropic drugs in nursing homes’, Am Fam Physician. 1:61 (5):1437–46, available at https://pubmed.ncbi.nlm.nih.gov/10735348/, accessed 15 February 21
Harris, M., C. Aschkenasi, C. Elias, A. Chandrankunnel, E. Nillni, C. Bjørbæk, J. Elmquist, J. Flier and A. Hollenberg (2001), ‘Transcriptional regulation of the thyrotropin-releasing hormone gene by leptin and melanocortin signaling’, Journal Of Clinical Investigation, 107 (1), 111–20, available at https://www.jci.org/articles/view/10741, accessed 1 May 2020
Harris, R. (2014), ‘Direct and indirect effects of leptin on adipocyte metabolism’, Biochimica Et Biophysica Acta (BBA) – Molecular Basis Of Disease, 1842 (3), 414–23, available at https://www.sciencedirect.com/science/article/pii/S0925443913001634, accessed 5 June 2020
Hays, N. and S. Roberts (2006), ‘The anorexia of aging in humans’, Physiology and Behavior, 88 (3), 257–66, available at https://www.sciencedirect.com/science/article/abs/pii/S0031938406002381, accessed 4 June 2020
Huerta A., S. Navas-Carretero, P. Prieto-Hontoria, J. Martínez and M. Moreno-Aliaga (2015), ‘Effects of α-lipoic acid and eicosapentaenoic acid in overweight and obese women during weight loss’, Obesity (Silver Spring), 23 (2), 313–21, available at https://pubmed.ncbi.nlm.nih.gov/25594166/, accessed 18 August 2020
Jackson, S.,, F. Leahy, S. Jebb, A. Prentice, W. Coward and L. Bluck (2007), ‘Frequent feeding delays the gastric emptying of a subsequent meal’, Appetite. 48 (2), 199–205
Josic J., A. Olsson, J. Wickeberg, S. Lindstedt and J. Hlebowicz (2010), ‘Does green tea affect postprandial glucose, insulin and satiety in healthy subjects: A randomized controlled trial’, Nutr. J, 9 (63), available at https://pubmed.ncbi.nlm.nih.gov/21118565/, accessed 12 February 21
Kappeler, L., D. Gourdji, P. Zizzari, M. Bluet-Pajot and J. Epelbaum (2003), ‘Age-associated changes in hypothalamic and pituitary neuroendocrine gene expression in the rat’, Journal Of Neuroendocrinology, 15 (6), 592–601, available at https://onlinelibrary.wiley.com/doi/abs/10.1046/j.1365-2826.2003.01039.x, accessed 30 June 2020
Kalra, S., M. Dube, A. Sahu, C. Phelps and P. Kalra (1991), ‘Neuropeptide Y secretion increases in the paraventricular nucleus in association with increased appetite for food’, Proc. Natl. Acad. Sci, 88, 10931–35, available at https://www.pnas.org/content/88/23/10931.short, accessed 18 August 2020
Kalson, N., F. Hext, A. Davies, C. Chan, A. Wright and C. Imray (2010), ‘Do changes in gastro-intestinal blood flow explain high-altitude anorexia?’, European Journal of Clinical Investigation, 40 (8), 735–41
Kayser, B., and S. Verges (2013), ‘Hypoxia, energy balance and obesity: From pathophysiological mechanisms to new treatment strategies’, Obes. Rev., 14, 579–92
Kietzmann, T. and V. Mäkelä (2021), ‘The hypoxia response and nutritional peptides’, Peptides, 170507, available at https://www.sciencedirect.com/science/article/pii/S0196978121000152, accessed 14 February 21
Kmiec, Z. (2006). ‘Central regulation of food intake in ageing’. J Physiol Pharmacol, 57, 7-16, available at https://pubmed.ncbi.nlm.nih.gov/17228084/, accessed 3 August 2020
Koch L (2013) ‘Growth hormone in health and disease: Novel ghrelin mimetic is safe and effective as a GH stimulation test’, Nat Rev Endocrinol, 9, 315, available at https://www.nature.com/articles/nrendo.2013.89, accessed 18 August 2020
Kong, W., N. Martin K. Smith, J. Gardiner, I. Connoley, D. Stephens, W. Dhillo, M. Ghatei, C. Small and S. Bloom (2004), ‘Triiodothyronine stimulates food intake via the hypothalamic ventromedial nucleus independent of changes in energy expenditure’, Endocrinology, 145 (11), 5252–58, available at https://academic.oup.com/endo/article/145/11/5252/2500746, accessed 2 May 2020
Lanni A., L. Beneduce, A. Lombardi, M. Moreno, O. Boss, P. Muzzin, J. Giacobino and F. Goglia (1999), ‘Expression of uncoupling protein-3 and mitochondrial activity in the transition from hypothyroid to hyperthyroid state in rat skeletal muscle’, FEBS Lett, 444 (2–3), 250–54., available at https://pubmed.ncbi.nlm.nih.gov/10050769/, accessed 15 February 21
Lee, P. and J. Dixon (2017), ‘Food for thought: Reward mechanisms and hedonic overeating in obesity’, Curr Obes Rep 6, 353–61
Lippl, F., S. Neubauer, S. Schipfer, N. Lichter, A. Tufman, B. Otto and R. Fischer (2010), ‘Hypobaric hypoxia causes body weight reduction in obese subjects’, Obesity, 18 (4), 675–81
Madelaine, R., K. Ngo, G. Skariah and P. Mourrain (2020), ‘Genetic deciphering of the antagonistic activities of the melanin-concentrating hormone and melanocortin pathways in skin pigmentation’, PLOS Genetics, 16 (12), 1009244, available at https://journals.plos.org/plosgenetics/article?id=10.1371/journal.pgen.1009244, accessed 12 February 21
Mani, B., N. Puzziferri, Z. He, J. Rodriguez, S. Osborne-Lawrence, N. Metzger, N. China, B. Gaylinn, M. Thorner, E. Thomas, J. Bell, K. Williams, A. Goldstone and J. Zigman (2019), ‘LEAP2 changes with body mass and food intake in humans and mice’, Journal of Clinical Investigation, 129 (9), 3909–23
Matu, J., K. Deighton, T. Ispoglou and L. Duckworth, L (2017), ‘The effect of moderate versus severe simulated altitude on appetite, gut hormones, energy intake and substrate oxidation in men’, Appetite, 113, 284–92
Mayo Clinic (2021), Myocardial ischemia - Diagnosis and treatment, available at https://www.mayoclinic.org/diseases-conditions/myocardial-ischemia/diagnosis-treatment/drc-20375422, accessed 15 February 2021
Mekjavic, I., M. Amon, R. Kölegård, S. Kounalakis, L. Simpson, O. Eiken, M. Keramidas and I. Macdonald (2016), ‘The effect of normobaric hypoxic confinement on metabolism, gut hormones, and body composition’, Frontiers in Physiology, 7, available at https://www.frontiersin.org/articles/10.3389/fphys.2016.00202/full, accessed 14 February 21
Méquinion, M., C. Foldi and Z. Andrews (2020), ‘The ghrelin-agrp neuron nexus in anorexia nervosa: implications for metabolic and behavioral adaptations’, Frontiers in Nutrition, 6, available at https://doi.org/10.3389/fnut.2019.00190, accessed 12 February 21
Moriguti, J., S. Das, E. Saltzman, A. Corrales, M. McCrory, A. Greenberg and S. Roberts (2000), ‘Effects of a 6-week hypocaloric diet on changes in body composition, hunger, and subsequent weight regain in healthy young and older adults’, The Journals of Gerontology Series A: Biological Sciences And Medical Sciences, 55 (12), B580–B587, available at https://academic.oup.com/biomedgerontology/article/55/12/B580/555927, accessed 23 May 2020
Morley, J. (2001), ‘Decreased food intake with aging’, The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 56 (Supplement 2), 81–88, available at https://academic.oup.com/biomedgerontology/article/56/suppl_2/81/581099, accessed 4 July 2020
Morton, G., D. Cummings, D. Baskin, G. Barsh and M. Schwartz, M (2006), ‘Central nervous system control of food intake and body weight’, Nature, 443 (7109), 289–95
Mullur, R., Y. Liu and G. Brent (2014), ‘Thyroid hormone regulation of metabolism’, Physiological Reviews, 94 (2), 355–82
Netzer N., R. Chytra and T. Kupper T (2008), ‘Low intense physical exercise in normobaric hypoxia leads to more weight loss in obese people than low intense physical exercise in normobaric sham hypoxia’, Sleep Breath, 12, 129–34, available at Netzer2008_Article_LowIntensePhysicalExerciseInNo.pdf, accessed14 February 21
NHANES (2011–2012): ‘Taste & smell: Data documentation, codebook, and frequencies’, available at https://wwwn.cdc.gov/Nchs/Nhanes/2011-2012/CSQ_G.htm, accessed 15 February 21
NHANES (2013–2014): ‘Taste & smell: data documentation, codebook, and frequencies’, available at https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/CSQ_H.htm, accessed 15 February 21
Obici S., Z. Feng, A. Arduini, R. Conti and L. Rossetti (2003), ‘Inhibition of hypothalamic carnitine palmitoyltransferase-1 decreases food intake and glucose production’, Nat Med. 9 (6), 756–61, available at Inhibition of hypothalamic carnitine palmitoyltransferase-1 decreases food intake and glucose production - PubMed (nih.gov), accessed 14 February 21
Okumura, T. and T. Nozu (2011), ‘Role of brain orexin in the pathophysiology of functional gastrointestinal disorders’, J. Gastroenterol. Hepatol. 26 (Suppl. 3), 61–66
O’Leary, L. (2014), ‘Orexin and melanin-concentrating hormone neurons: A hypothalamic interface for sleep and feeding regulation’, Bioscience Horizons, 7 (0), hzu008–hzu008, available at https://academic.oup.com/biohorizons/article/doi/10.1093/biohorizons/hzu008/242885, accessed 30 April 2020
Paeger, L., I. Karakasilioti, J. Altmüller, P. Frommolt, J. Brüning and P. Kloppenburg (2017), ‘Antagonistic modulation of NPY/AgRP and POMC neurons in the arcuate nucleus by noradrenalin’, eLife, 6, available at https://pubmed.ncbi.nlm.nih.gov/28632132/, accessed 12 February 21
PDR Search. (2020), ‘Megace (megestrol acetate) dose, indications, adverse effects, interactions’, available at PDR.net, 2020, www.pdr.net/drug-summary/Megace-megestrol-acetate-890.343, accessed 17 August, 2020
Perello, M., T. Friedman, V. Paez-Espinosa, X. Shen, R. Stuart and E. Nillni (2006), ‘Thyroid hormones selectively regulate the posttranslational processing of prothyrotropin-releasing hormone in the paraventricular nucleus of the hypothalamus’, Endocrinology, 147 (6), 2705–16, available at https://academic.oup.com/endo/article/147/6/2705/2878713, accessed 4 May 2020
Persons, R. and W. Nichols (2007), ‘Should we use appetite stimulants for malnourished elderly patients?’, The Journal of Family Practice, 56 (9), 761–62, available at https://www.mdedge.com/familymedicine/article/62827/geriatrics/should-we-use-appetite-stimulants-malnourished-elderly, accessed 29 July 2020
Porkka-Heiskanen, T., L. Alanko, A. Kalinchuk, S. Heiskanen and D. Stenberg (2004), ‘The effect of age on prepro-orexin gene expression and contents of orexin A and B in the rat brain’, Neurobiology Of Aging, 25 (2), 231–38, available at https://www.sciencedirect.com/science/article/abs/pii/S0197458003000435, accessed 20 May 2020
Puigserver, P. (2005), ‘Tissue-specific regulation of metabolic pathways through the transcriptional coactivator PGC1–alpha’, Int J Obes (Lond)., 29 Suppl 1:S5–9., available at https://pubmed.ncbi.nlm.nih.gov/15711583/, accessed 15 February 21
Quintero, P., F. Milagro, J. Campion and J. Martinez (2010), ‘Impact of oxygen availability on body weight management’, Med.Hypotheses, 74, 901–07
Reseland J., S. Anderssen, K. Solvoll, I. Hjermann, P. Urdal, I. Holme and C. Drevon (2001), ‘Effect of long-term changes in diet and exercise on plasma leptin concentrations’, Am J Clin Nutr, 73 (2), 240–45, available at https://pubmed.ncbi.nlm.nih.gov/11157319/, accessed 18 August 2020
Reuben D., S. Hirsch, K. Zhou and G. Greendale (2005), ‘The effects of megestrol acetate suspension for elderly patients with reduced appetite after hospitalization: A phase II randomized clinical trial’, J Am Geriatr Soc, 53, 970–75, available at https://pubmed.ncbi.nlm.nih.gov/15935019/, accessed 30 July 2020
Rigamonti, A., A. Pincelli, B. Corra, R. Viarengo, S. Bonomo, D. Galimberti, M. Scacchi, E. Scarpini, F. Cavagnini and E. Muller (2002), ‘Plasma ghrelin concentrations in elderly subjects: comparison with anorexic and obese patients’, Journal Of Endocrinology, 175 (1), R1–R5, available at https://joe.bioscientifica.com/view/journals/joe/175/1/R1.xml, accessed 20 May 2020
Rolls, B., K. Dimeo and D. Shide (1995), ‘Age-related impairments in the regulation of food intake’, The American Journal Of Clinical Nutrition, 62 (5), 923–31, available https://academic.oup.com/ajcn/article-abstract/62/5/923/4651765?redirectedFrom=fulltext, accessed 21 May 2020
Roy, M., P. Gaudreau P and H. Payette (2016), ‘A scoping review of anorexia of aging correlates and their relevance to population health interventions’, Appetite.105, 688–99, available at https://pubmed.ncbi.nlm.nih.gov/27374898/, accessed 10 January 2022
Schiffman S. (1983), ‘Taste and smell in disease’, N Engl J Med, 308, 1275–79
Schiffman, S. (1997), ‘Taste and smell losses in normal aging and disease’, JAMA: The Journal of the American Medical Association, 278 (16), 1357, available at https://jamanetwork.com/journals/jama/article-abstract/418447, accessed 30 May 2020
Schiffman S., Zervakis J. (2002). ‘Taste and smell perception in the elderly: Effect of medications and disease.’ Adv Food Nutr Res, 44, 247–346
Schiffman S. (2007), ‘Critical illness and changes in sensory perception’, Proc Nutr Soc., 66, 331–45
Schiffman S. and K. Rother (2013), ‘Sucralose, a synthetic organochlorine sweetener: Overview of biological issues’, J Toxicol Environ Health B Crit Rev, 16, 399–451
Scott, M., J. Lachey, S. Sternson, C. Lee, C. Elias, J. Friedman J. Elmquist (2009), ‘Leptin targets in the mouse brain’, J Comp Neurol. 514 (5), 518–32
Shapiro A., M. Matheny, Y. Zhang, N. Tumer, K. Yan-Cheng, E. Rodrigues, S. Zolotukhin and P. Scarpace (2008), ‘Synergy between leptin therapy and a seemingly negligible amount of voluntary wheel running prevents progression of dietary obesity in leptin-resistant rats’, Diabetes, 57 (3), 614–22, available at https://pubmed.ncbi.nlm.nih.gov/18086903/, accessed 18 August 2020
Shimizu, H., H. Arima, M. Watanabe, M. Goto, R. Banno, I. Sato, N. Ozaki, H. Nagasaki and Y. Oiso (2008), ‘Glucocorticoids increase neuropeptide Y and agouti-related peptide gene expression via adenosine monophosphate-activated protein kinase signaling in the arcuate nucleus of rats,’ Endocrinology, 149 (9), 4544–53
Shukla, V., S. Singh, P. Vats, V. Singh, S. Singh and P. Banerjee (2005), ‘Ghrelin and leptin levels of sojourners and acclimatized lowlanders at high altitude’, Nutr Neurosci, 8,161–65
Silva, J. (2011), ‘Physiological importance and control of non-shivering facultative thermogenesis’, Frontiers in Bioscience, S3 (1), 352–71
Simmons, S., E. Keeler, X. Zhuo, K. Hickey, H. Sato and J. Schnelle (2008), ‘Prevention of unintentional weight loss in nursing home residents: A controlled trial of feeding assistance’, Journal of the American Geriatrics Society, 56 (8), 1466–73, available at https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1532-5415.2008.01801.x, accessed 1 August 2020
Snyder, E., R. Carr, C. Deacon and B. Johnson (2008), ‘Overnight hypoxic exposure and glucagon-like peptide-1 and leptin levels in humans’, Appl Physiol Nutr Metab, 33, 929–35
Soenen, S., C. Rayner, M. Horowitz and K. Jones (2015), ‘Gastric emptying in the elderly’, Clin Geriatr Med. 31 (3), 339–53, available at https://pubmed.ncbi.nlm.nih.gov/26195094/, accessed 11 January 2022
Spiegel K., R. Leproult, M. L’hermite-Balériaux., G. Copinschi, P. Penev and E. Van Cauter (2004), ‘Leptin levels are dependent on sleep duration: Relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin’, J Clin Endocrinol Metab, 89 (11), 5762–71, available at https://pubmed.ncbi.nlm.nih.gov/15531540/, accessed 18 August 2020
Squecco, R., R. Garella, G. Luciani, F. Francini and M. Baccari (2011), ‘Muscular effects of orexin A on the mouse duodenum: Mechanical and electrophysiological studies’, The Journal of Physiology, 589 (21), 5231–46, available at https://physoc.onlinelibrary.wiley.com/doi/full/10.1113/jphysiol.2011.214940, accessed 4 July 2020
Strominger, J. and J. Brobeck (1953) ‘A mechanism of regulation of food intake’, Yale Journal Of Biology And Medicine, 25 (5), 383–90.
Sturm, K., B. Parker, J. Wishart, C. Feinle-Bisset, K. Jones, I. Chapman and M. Horowitz (2004). ‘Energy intake and appetite are related to antral area in healthy young and older subjects’, The American Journal of Clinical Nutrition, 80 (3), 656–67, available at https://academic.oup.com/ajcn/article/80/3/656/4690544, accessed 24 May 2020
Tai, K., D. Gentilcore, K. Jones, L. Banh, O. Gilja, A. Hammond, C. Feinle-Bisset, M. Horowitz and I. Chapman (2011), ‘Orlistat accentuates the fat-induced fall in blood pressure in older adults’, Br J Nutr., 106 (3), 417–24
Takeda, H., S. Muto, T. Hattori, C. Sadakane, K. Tsuchiya, T. Katsurada, T. Ohkawara, N. Oridate and M. Asaka (2010), ‘Rikkunshito ameliorates the aging-associated decrease in ghrelin receptor reactivity via phosphodiesterase III inhibition’, Endocrinology, 151 (1), 244–52, available at https://academic.oup.com/endo/article/151/1/244/2456117, accessed 3 July 2020
Tieken, S., H. Leidy, A. Stull, R. Mattes, R. Schuster and W. Campbell (2007). ‘Effects of solid versus liquid meal-replacement products of similar energy content on hunger, satiety, and appetite-regulating hormones in older adults’, Hormone and metabolic research, 39 (5), 389–94
Uchida, Y., C. Tsunekawa and I. Sato (2020), ‘Systemic acyl-ghrelin increases tail skin temperature in rats without affecting their thermoregulatory behavior in a cold environment’, Neuroscience Letters, 737, 135306, available at https://pubmed.ncbi.nlm.nih.gov/32822766/, accessed 12 February 21
Uriarte, M., P. De Francesco, G. Fernandez, A. Cabral, D. Castrogiovanni, T. Lalonde, L. Luyt, S. Trejo and M. Perello (2018), ‘Evidence supporting a role for the blood-cerebrospinal fluid barrier transporting circulating ghrelin into the brain’, Molecular Neurobiology, 56 (6), 4120–34
Van der Ploeg, L., H. Laken, S. Sharma, R. Datta, H. Halem, J. Dong, C. Touvay, M. Teillot, P. Noonan, L. Tartaglia, L. Stoner, B. Henderson, K. Gottesdienr and M. Culler (2014), ‘Preclinical gastrointestinal prokinetic efficacy and endocrine effects of the ghrelin mimetic RM-131’, Life Sci, 109, 20–29, available at https://pubmed.ncbi.nlm.nih.gov/24931905/, accessed 18 August 2020
Varela, L., S. Suyama, Y. Huang, M. Shanabrough, M. Tschöp, X. Gao, F. Giordano and T. Horvath (2017), ‘Endothelial HIF-1α enables hypothalamic glucose uptake to drive pomc neurons’, Diabetes, 66 (6),1511–20
Wasinski, F., I. Furigo, P. Teixeira, A. Ramos-Lobo, C. Peroni, P. Bartolini, E. List, J. Kopchick and J. Donato (2020), ‘Growth hormone receptor deletion reduces the density of axonal projections from hypothalamic arcuate nucleus neurons’, Neuroscience, 434,136–47
Wasse, L., C. Sunderland, J. King, R. Batterham and D. Stensel (2012), ‘Influence of rest and exercise at a simulated altitude of 4,000 m on appetite, energy intake, and plasma concentrations of acylated ghrelin and peptide YY’, J.Appl.Physiol, (112) 552–59
Wauman, J., L. Zabeau and J. Tavernier (2017), ‘The leptin receptor complex: Heavier than expected?’, Frontiers in Endocrinology, 8
Weitzel, J., C. Radtke and H. Seitz (2001), ‘Two thyroid hormone-mediated gene expression patterns in vivo identified by cDNA expression arrays in rat’, Nucleic Acids Res., 29 (24), 5148–55, available at https://pubmed.ncbi.nlm.nih.gov/11812848/, accessed 15 February 21
Wernette, C., B. White and C. Zizza (2011), ‘Signaling proteins that influence energy intake may affect unintentional weight loss in elderly persons’, Journal of the American Dietetic Association, 111 (6), 864–73, available at https://pubmed.ncbi.nlm.nih.gov/21616199/, accessed 4 July 2020
Westerterp, K, and B. Kayser (2006), ‘Body mass regulation at altitude’, Eur.J.Gastroenterol.Hepatol., 18,1–3
Yeh S., S. Wu, T. Lee, J. Olson, M. Stevens, T. Dixon, R. Porcelli and M. Schuster (2001), ‘Improvement in quality-of-life measures and stimulation of weight gain after treatment with megestrol acetate or suspension in geriatric cachexia: results of a double-blind, placebo controlled study’, J Am Geriatr Soc, 48, 485–92, available at https://pubmed.ncbi.nlm.nih.gov/10811540/, accessed 30 July 2020
Yoshihara, T., S. Honma and K. Honma (1996), ‘Effects of restricted daily feeding on neuropeptide Y release in the rat paraventricular nucleus’, Am. J. Physiol, 270, E589–E595, available at https://journals.physiology.org/doi/abs/10.1152/ajpendo.1996.270.4.E589, accessed 18 August 2020
Zamboni, M., E. Zoico, F. Fantin, M. Panourgia, V. Di Francesco, P. Tosoni, B. Solerte, R. Vettor, and O. Bosello (2004), ‘Relation between leptin and the metabolic syndrome in elderly women’, The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 59 (4), M396–M400, available at https://academic.oup.com/biomedgerontology/article/59/4/M396/637791, accessed 30 May 2020
Zhang, Y., M. Matheny, N. Tümer and P. Scarpace (2004), ‘Aged-obese rats exhibit robust responses to a melanocortin agonist and antagonist despite leptin resistance’, Neurobiology Of Aging, 25 (10), 1349–60, available at https://www.sciencedirect.com/science/article/abs/pii/S0197458004001125, accessed 30 June 2020
Zhang, H., G. Zhang, G. Gonzalez,, S. Park and D. Cai (2011), ‘Hypoxia-inducible factor directs POMC gene to mediate hypothalamic glucose sensing and energy balance regulation’, PLoS Biol., 9, available at https://journals.plos.org/plosbiology/article?id=10.1371/journal.pbio.1001112, accessed 14 February 21
Zhang, J., X. Li, Y. Zhou, L. Cui, J. Li, C. Wu, Y. Wan, J. Li, and Y. Wang (2017), ‘The interaction of MC3R and MC4R with MRAP2, ACTH, α-MSH and AgRP in chickens’, Journal of Endocrinology, 234 (2),155–74
α-MSH: biologically active peptide of POMC; ligand for melanocortin receptors; inhibited by MCH and AgRP neuropeptides
AgRPARC: refers to the orexigenic neuronal subset of the ARC which contains AgRP, NPY, and GABA neurotransmitters; green light
AgRP: an orexigenic neurotransmitter located in the AgRPARC neuronal subset; an inhibitor of α-MSH
Anorexia of ageing: declined food intake with age
ARC: located in the hypothalamus near the median eminence, this brain region contains two distinct neuronal subsets thought to control hunger and satiation
Cachexia: weakness and wasting of the body due to severe chronic illness
CART: an anorectic neurotransmitter located in one of the neuronal subsets of the ARC
Chemosensory: (of a sense organ or receptor) responsive to chemical stimuli
CPT-1: enzyme in the outer mitochondrial membrane that converts long-chain acyl-CoA species to their corresponding long-chain acyl-carnitines for transport into the mitochondria
Duodenum: the first part of the small intestine immediately beyond the stomach, leading to the jejunum
Endogenous: growing or originating from within an organism
Enteric: relating to intestines
Enteric plexus: a complex autonomic nerve plexus (bundle of nerves and vessels) inside the walls of the gastrointestinal tract, from oesophagus to anus
GABA: inhibitory neurotransmitter
Ghrelin: hunger hormone released from endocrine cells; activates AgRPARC neurons; main controller of orexigenic pathway
Lateral hypothalamus: portion of the brain associated with the orexigenic pathway; contains ORXLHA and MCHLHA
Leptin: satiation hormone released from white adipocytes; activates POMCARC neurons; inhibits AgRPARC neurons’ main controller of anorectic pathway
MCHLHA: melanin-concentrating hormone; antagonist for α-MSH; activated by ORX neuropeptides; orexigenic neurons
MCR: main receptor utilised in the anorectic pathway; α-MSH is the predominant ligand
Morbidity: the condition of being diseased
Neuropeptide: a compound containing two or more amino acids in which the carboxyl group of one acid is linked to the amino group of the other; a neuropeptide would be produced in the brain
NPY: an orexigenic neurotransmitter located in one of the neuronal subsets in the ARC
Ob-Rb: leptin receptor
ORXLHA: orexin neurons located in the lateral hypothalamus; ORX neuropeptides enhance gut motility; activated by NPY; orexigenic neurons
Paraventricular nucleus: portion of the brain that predominantly contains anorectic neuronal subsets such as TRH and CRH; innervated by POMCARC neurons
Peptide: a compound containing two or more amino acids in which the carboxyl group of one acid is linked to the amino group of the other; a neuropeptide would be produced in the brain
POMC: an anorectic neurotransmitter located in the POMCARC neuronal subset
POMCARC:refers to the anorectic neuronal subset of the ARC which contains POMC and CART neurotransmitters: red light
Post-prandial: of or relating to a meal (before or after)
Pre-prandial: of or relating to a meal (before or after)
Sarcopenia: loss of muscle tissue as a natural part of the ageing process
T3: biologically active thyroid hormone; associated with the regulation of the body’s metabolic rate
TRHPVN: activation results in a downstream signalling cascade culminating in the production of T3
UCP: proton channel found in inner mitochondrial membrane that uncouple oxidative phosphorylation from ATP, thereby generating heat as a byproduct
To cite this paper please use the following details: Plotkin, A.P. (2022), 'Appetite Control With Ageing: A Narrative Review Focused on the POMC and AgRP Neurons', Reinvention: an International Journal of Undergraduate Research, Volume 15, Issue 1, https://reinventionjournal.org/article/view/707. Date accessed [insert date]. If you cite this article or use it in any teaching or other related activities please let us know by e-mailing us at Reinventionjournal@warwick.ac.uk.