
Molly Norah Lavery, Conor Francis Hunter Murphy and Emma Kate Bowman, Monash University, Australia
Ophiocordyceps is a genus of pathogenic fungi that predominantly parasitises insects of the tropics. While there is considerable research derived from alternative tropical regions, there is limited documentation of Ophiocordyceps fungi in Borneo. This paper investigates the spatial dynamics of zombie ant graveyards – a manifestation of the Ophiocordyceps unilateralis species – and explores the optimal height for spore dispersal in a Bornean rainforest. In the present study, an area of Gunung Mulu National Park was searched for O. unilateralis-infected ants. Once an infected ant had been located, the surrounding area was methodically searched to allow for the height and location of all surrounding ants to be recorded. Infected ants were found at variable heights between the four sites (means of 28.9–57.6cm), which was above the expected height laid out in similar studies (approximately 25cm). It is suggested that these heights may correspond to locations at which temperature and humidity are optimal for spore dispersal and fungal growth, and that these heights differ depending on unique features of the environment.
Keywords: Ophiocordyceps unilateralis, host-parasite interaction, entomopathogenic fungi, formicidae pathogen, fungal behavioural manipulation, Ophiocordycipitaceae
Tropical regions are characterised by their high temperature and rainfall, with limited seasonality, as well as their remarkably high diversity (Brown, 2014). The Latitudinal Diversity Gradient (LDG) describes this phenomenon whereby biodiversity increases with lower latitudes (Jablonski et al., 2006). Numerous hypotheses have been proposed to explain the LDG, with research largely suggesting that tropical conditions result in high productivity, thus allowing for a higher species richness to be maintained as more species are able to obtain sufficient resources (Brown, 2014; Fittkau and Klinge, 1973; Phillips et al., 1994). This high density and diversity of organisms seen in the tropics is particularly notable in the case of invertebrates, with researchers observing a significantly higher density of invertebrates per unit area relative to temperate and polar regions (Fittkau and Klinge, 1973). Ants, specifically, are found at their highest densities in tropical rainforests, making up approximately 25 per cent of rainforest biomass, with density estimates ranging from two to thirty nests per square metre (Baccaro and Ferraz, 2013: 103; Fittkau and Klinge, 1973: 11). While ants appear to thrive in tropical rainforests, so too do many ant parasites. Ants are parasitised by invertebrates such as Polyergus breviceps, trematodes including Dicrocoelium dendriticum, and fungi such as Ophiocordyceps (Araújo et al., 2018; Martín-Vega et al., 2018; Torres and Tsutsui, 2016).
Parasitism involves the physical takeover of a host individual and, in specific cases, can culminate in behavioural manipulation (Lefevre et al., 2009; Poulin, 2000). These behaviours are extended phenotypes of the parasite that result in the increased fitness of the parasitic individual, which is detrimental to the host (Andersen et al., 2009; Andersen and Hughes, 2012; Dawkins, 1982; Thomas et al., 2005). The obligate fungal parasitoid Ophiocordyceps is a genus of particularly successful ant parasites, capable of wiping out entire colonies of over 10,000 individuals, and is an extreme example of adaptive manipulation (Andersen et al., 2009; Evans and Samson, 1982: 445; Burchill and Moreau, 2016: 293; Evans et al., 2011: 600). Ophiocordyceps fungi are widespread within tropical forest environments, but are largely uncommon within temperate ecosystems (Araújo et al., 2018; Pontoppidan et al., 2009). It has been suggested that this pattern of distribution results from the fungi’s requirements for the high temperatures and humidity typical of tropical rainforests (Evans et al., 2011).
Ophiocordyceps directly infects worker ants while they are foraging on the forest floor. Spores from previously infected ants litter the ground and, when they are walked across, adhere to and subsequently penetrate the ant’s cuticle (Pontoppidan et al., 2009: 2). Once infected, ants begin to walk and climb in random directions in place of their typical coordinated movements (Hughes et al., 2011: 4), earning them the name ‘zombie ants’. They then repeatedly convulse, frequently causing them to fall to the forest floor (Hughes et al., 2011: 6). The infected ants become increasingly disoriented over the following three to six days and ultimately ascend into the vegetation (Pontoppidan et al., 2009: 2). Immediately prior to being killed by the fungus, the disoriented ants will perform the ‘death grip’, which involves the infected ant biting into vegetation permanently via their mandibles (Andersen et al., 2009: 424). This ‘death grip’ results in the atrophy of the ant’s mandibular muscles, preventing it from releasing its grip, thereby leading to its immobilisation and eventual death (Hughes et al., 2011: 8). The fungi’s manipulation of the hosts’ behaviour is, in effect, an expression of fungal behaviour as prescribed in the fungal genome, and is designed to optimise the subsequent spread of infection (Andersen et al., 2009; Araújo et al., 2018). Despite aerial spore dispersal, the immobilisation and eventual death of ants are typically undertaken in areas where the cadavers of previously manipulated ants are abundant, leading to the formation of ant graveyards (Pontoppidan et al., 2009). It is within these graveyards that the infecting fungus has been seen to grow; the stroma and perithecial plate of the fungus emerge from the ant’s head soon after it dies (Figure 1), dispersing spores that go on to infect additional individuals and colonies (de Bekker et al., 2014). The spatial distribution of graveyards consisting of the host genus Camponotus appear to accommodate the fungal parasite within a stable microclimatic niche suitable to support fungal growth and aerial spore dispersal (Andersen et al., 2009, Hughes et al., 2011). This spatial niche of infected ants is distinct from that of the healthy ants. These environments are highly specific: typically, between 94 and 95 per cent humidity, 20 to 30 °C, and on the underside of northward facing leaves (Andersen et al., 2009: 428). Andersen and colleagues (2009: 424) suggest that outside of this optimal zone, parasite fitness is significantly lower in terms of spore development and transmission potential, and this is a driver of the dense culmination of zombie ant graveyards in ideal locations (Evans, 1974; Pontoppidan et al., 2009). Once spores are released, they are deposited on the forest floor, where they lose infectiousness quickly; thus, infection must occur soon after dispersal (Sobczak et al., 2017: 1262). Pontoppidan et al., (2009: 5) suggests that foraging host ants of this species actively avoid graveyards, meaning that infection rates may be low without effective aerial dispersal, highlighting the importance of host manipulation and optimal relocation. The knowledge we have of the interaction between O. unilateralis and C. leonardi has resulted primarily from recent studies of zombie ants within Thai rainforests (Andersen et al., 2009; Hughes et al., 2011). The zombie ant communities in the Bornean rainforests of Sarawak are yet to be explored, and thus this study aims to investigate the characteristics of these communities. Consequently, we aim to build on the previous findings and determine the optimal height for O. unilateralis spore dispersal in Bornean rainforest conditions. Additionally, we aim to re-examine the effect of biotic and abiotic factors on the distribution of O. unilateralis-infected Camponotus leonardi ants and assess which factors influence the density and distribution of infected ants. By recording environmental conditions and the presence and density of infected ants, we endeavour to establish the typical distribution of Ophiocordyceps-infected ants within Gunung Mulu National Park, Borneo. It is hypothesised that there will be optimal conditions for zombie-ant occurrence as dictated by the Ophiocordyceps genome.

The study was conducted in a tropical dipterocarp forest within Gunung Mulu National Park in Sarawak, Borneo (over 528 km2) at an altitude of approximately 50m. The study was undertaken on 26 and 27 September at the beginning of the wet season (average September temperature approximately 30°C and average September precipitation approximately 285mm).
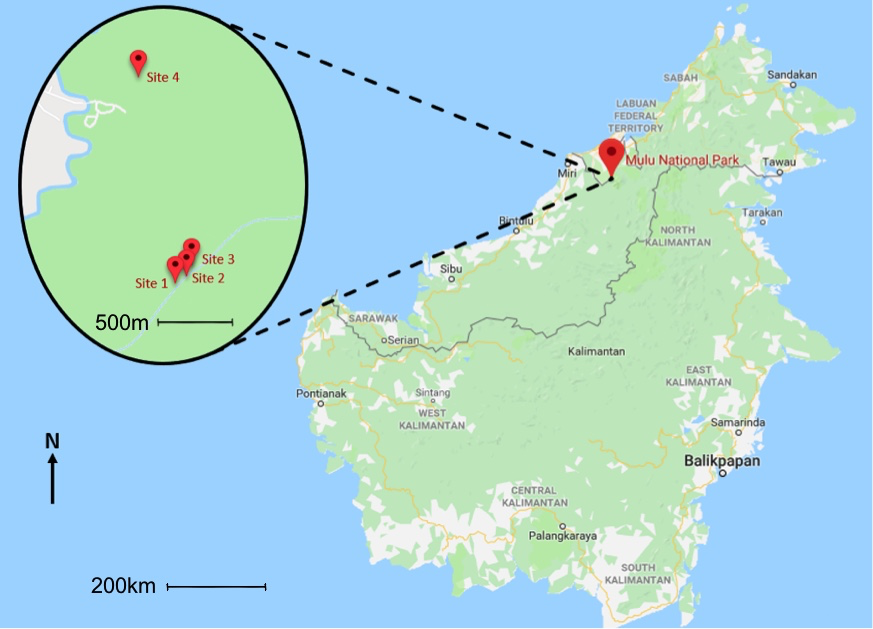
Sites were identified by inspecting Ophiocordyceps-infected ants on the undersides of low-hanging leaves located along select walking paths throughout the forest. Three sites were located on the Paku Valley Walk (Site 1 at 4.03109, 114.81765; Site 2 at 4.03144, 114.81818; Site 3 at 4.003210, 114.81863) and one on the Night Walk (Site 4 at 4.04356, 114.81516) (Figure 2). All sites were classified as dipterocarp forests, composed of multiple vegetation types. Site 2 and 3 had dense understories with sparse canopies, while Sites 1 and 4 had sparser understories with dense canopies. Once an infected ant had been identified, the surrounding leaves were checked within a 5-metre radius for additional infected ants. If additional ants were located in the immediate area, the coordinates were taken and flags were placed to mark the site.
Quadrats of 1m2 were searched outwards from the initially located ant at point (0, 0) extending in northern, eastern, southern and western directions. Each quadrat was marked out using raffia tape to clearly define its boundary. Every shrub, tree or other plant in that quadrat was carefully inspected for infected ants. Each leaf was gently overturned to ensure both surfaces of the leaves were searched, and all surfaces of plant stems and trunks were examined. The entire area of the quadrat was inspected up to a height that could no longer be reached without further equipment; this corresponded to a maximum height of approximately two metres. During the search, a few individual ants were observed at heights beyond this but were not included in the dataset as they were very uncommon and difficult to measure accurately. The location of each infected ant was recorded, in addition to its height above the ground. If one or more infected ants were located in a quadrat, the two subsequent quadrats in all directions were searched, until two successive quadrats were empty; this was considered the edge of the graveyard. Three sample specimens were retained and identified as ants of the Camponotus leonardi species, infected by Ophiocordyceps unilateralis. Subsequent ants were compared to these identified specimens, and it was concluded all graveyards consisted of the same ant species infected by the same parasitic fungus.
Environmental conditions at each site were measured and recorded. This included temperature, relative humidity taken at a height of ~1.5 m, wind speed at 10cm height intervals, and light levels. Light levels included PAR (Photosynthetically Active Radiation), PAR LAI (Leaf Area Index) and Sunflecks, and were measured using a ceptometer. PAR refers to the amount of sunlight that is intercepted, only taking into account light of wavelengths 400-700nm, as this is the portion of the light spectrum that plants utilise for energy via photosynthesis (Navrátil et al. 2007: 311). LAI is a measure of the total leaf area per unit of ground area; thus PAR LAI is a measure of how much usable light reaches the leaves, per unit area. Sunflecks are the small patches of intense light that briefly reach the ground level of vegetation. They occur when branches in the canopy sway, or the angle of the sun changes and allows small amounts of light to penetrate to the ground completely unobstructed. Measurements of temperature, wind speed and relative humidity were taken using a Kestrel meter once during the day and once at night. These measurements were taken at midday and midnight on the final day of data collection (27 September). Distance to the nearest water body was later measured using aerial images, as proximity to water, and thus a source of evaporation, may play an important role in humidity levels. Data was collated and analysis conducted using RStudio and Microsoft Excel (2019). The site map was created using Google Maps.
To determine patterns in the vertical distribution of ants within the reserve, the heights at which infected ants were found were compared across the four sites using independent samples t-tests. Kendall correlation analyses were performed to determine if there was a significant effect of temperature or humidity on the mean height of infected ants with a significance set at 0.05. In order to visually represent the spatial distribution of infected ants located at each site, density maps were created to illustrate the density of infected ants per quadrat in each site.
Following data collection, the four sites were compared in terms of environmental factors, the height at which ants were found, and their relative similarity to each other site. All sites were largely similar in all measured environmental conditions (temperature, relative humidity, wind speed, PAR, PAR LAI, Sunflecks; Table 1). Of particular interest was temperature and humidity, which were measured day and night using a Kestrel meter, with temperature varying by a maximum of 2 °C between sites and humidity by a maximum of 7 per cent (Table 1). Mean temperature across the sites was 29.78 °C (±0.14) during the day and 27.23 °C (±0.44) during the night. Mean humidity across sites was 90.05 per cent (±0.99) in the day and 90.01 per cent (±1.12) at night. Temperature and humidity measurements were taken at a single height; however, the study may have benefitted from these measurements being recorded at multiple points on a vertical gradient where variations may have been more obvious due to vertical vegetation structure.
The height of infected ants within each quadrat was measured up to a height of 2m with the mean and median ant height varying significantly between all sites except Sites 2 and 3 (Table 2, Figures 3 and 4). Sites were compared in terms of height by conducting six independent samples t-tests (Figure 3). The Kendall correlation analyses showed a significant but weak association of temperature and humidity on the mean height of infected ants across the four sites (Table 3). This indicates that temperature and humidity at each site significantly influences the mean height at which infected ants were found.
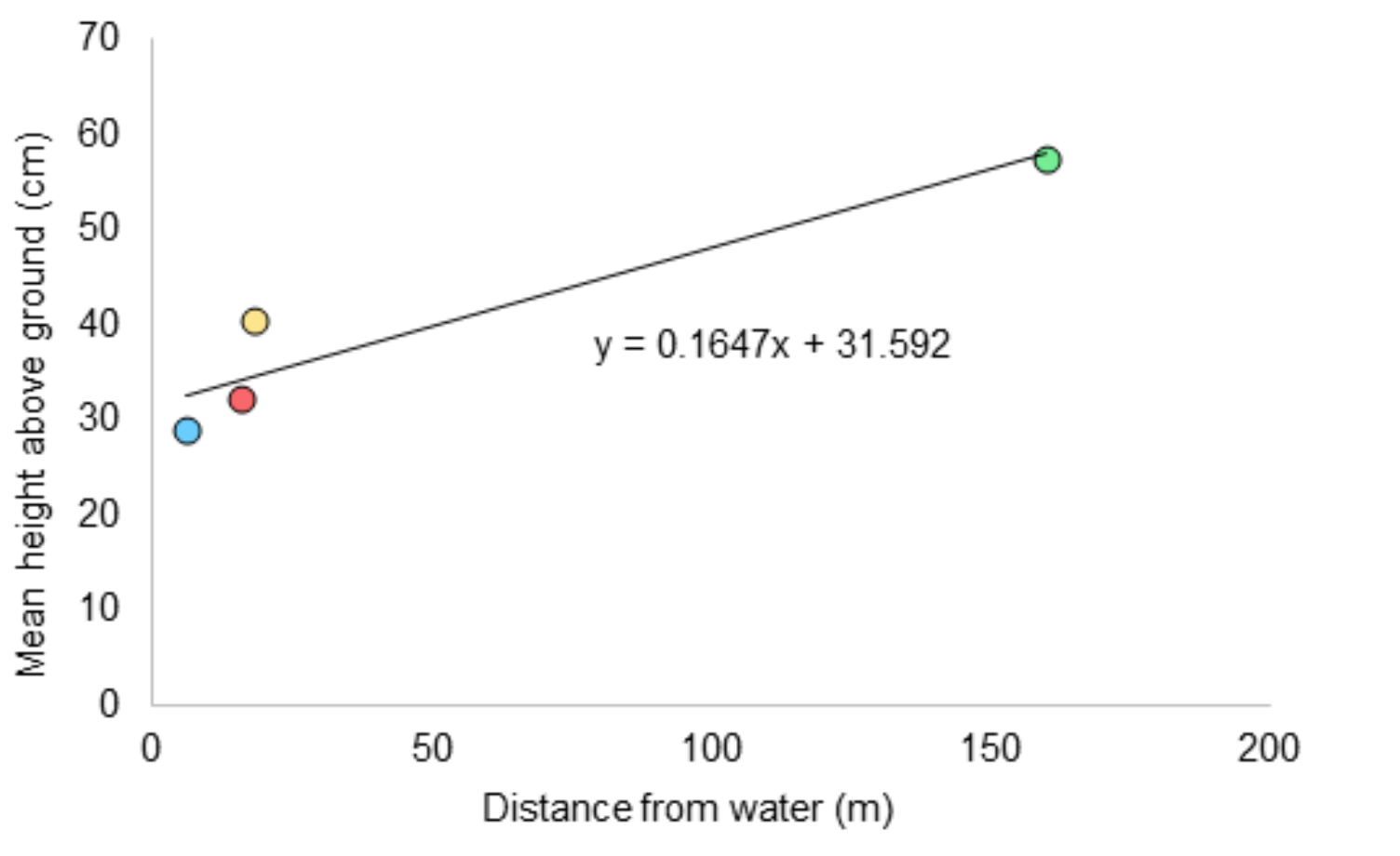
There was a positive trend between distance from water and average height above ground (R2=0.90; Figure 5); however, the speculated link to humidity gradients is not supported by the correlation analyses.
The majority of infected ants were found within a somewhat small vertical range across the four sites, with 75 per cent occurring below 55cm (Figure 4). This may indicate that the fungus favours certain heights due to host factors or environmental factors, which influence the fungi’s reproductive success. The density maps (Figure 6) illustrate the three-dimensional size and shape of each hot spot. Additionally, the density plots (Figure 7), generated using R Studio, illustrate the density variation between sites relative to height. Sites 2 and 3 show similar density variation across heights, peaking at approximately 25cm above ground, whereas Site 1 and Site 4 show a wider spread, peaking at approximately 30cm (Figure 7). This may be indicative of environmental differences, including geographical factors such as site location and distance from the nearest water body.
Table 1: Summary of environmental conditions at Sites 1–4 in Gunung Mulu National Park, Borneo. Wind speeds refer to the average wind speed at each site per time of day, measured on a vertical gradient of 0-200 cm at 10 cm intervals
|
Site 1 |
Site 2 |
Site 3 |
Site 4 |
|
|---|---|---|---|---|
|
Temperature – day (oC) |
29.1 |
29.2 |
28.6 |
29.5 |
|
Temperature – night (oC) |
26 |
27.2 |
27.9 |
27.8 |
|
Humidity – day (%) |
96.5 |
90.1 |
93 |
89.9 |
|
Humidity – night (%) |
91.8 |
92.4 |
88.2 |
87.9 |
|
Distance from body of water (m) |
18 |
16 |
6 |
160 |
|
Day wind (km/hr) |
0 |
0 |
0 |
0 |
|
Night wind (km/hr) |
0 |
0 |
0 |
0 |
|
PAR |
11.1 |
11.1 |
5.8 |
4.5 |
|
PAR LAI |
5.7 |
5.7 |
6.4 |
6.7 |
|
Sunflecks |
0 |
0 |
0 |
0 |
Table 2: Summary of graveyard characteristics at Sites 1–4 in Gunung Mulu National Park, Borneo
|
Site 1 |
Site 2 |
Site 3 |
Site 4 |
|
|---|---|---|---|---|
|
Number of ants |
59 |
20 |
176 |
292 |
|
Area (m2) |
45 |
22 |
124 |
299 |
|
Mean (±SE) ant height (cm) |
38.42 ± 2.45 |
28.9 ± 4.53 |
30.47 ± 1.14 |
57.78 ± 2.54 |
|
Median ant height (cm) |
35 |
24.5 |
27.5 |
45 |
|
Mean density (ants/m2) |
1.31 |
0.91 |
1.42 |
0.98 |
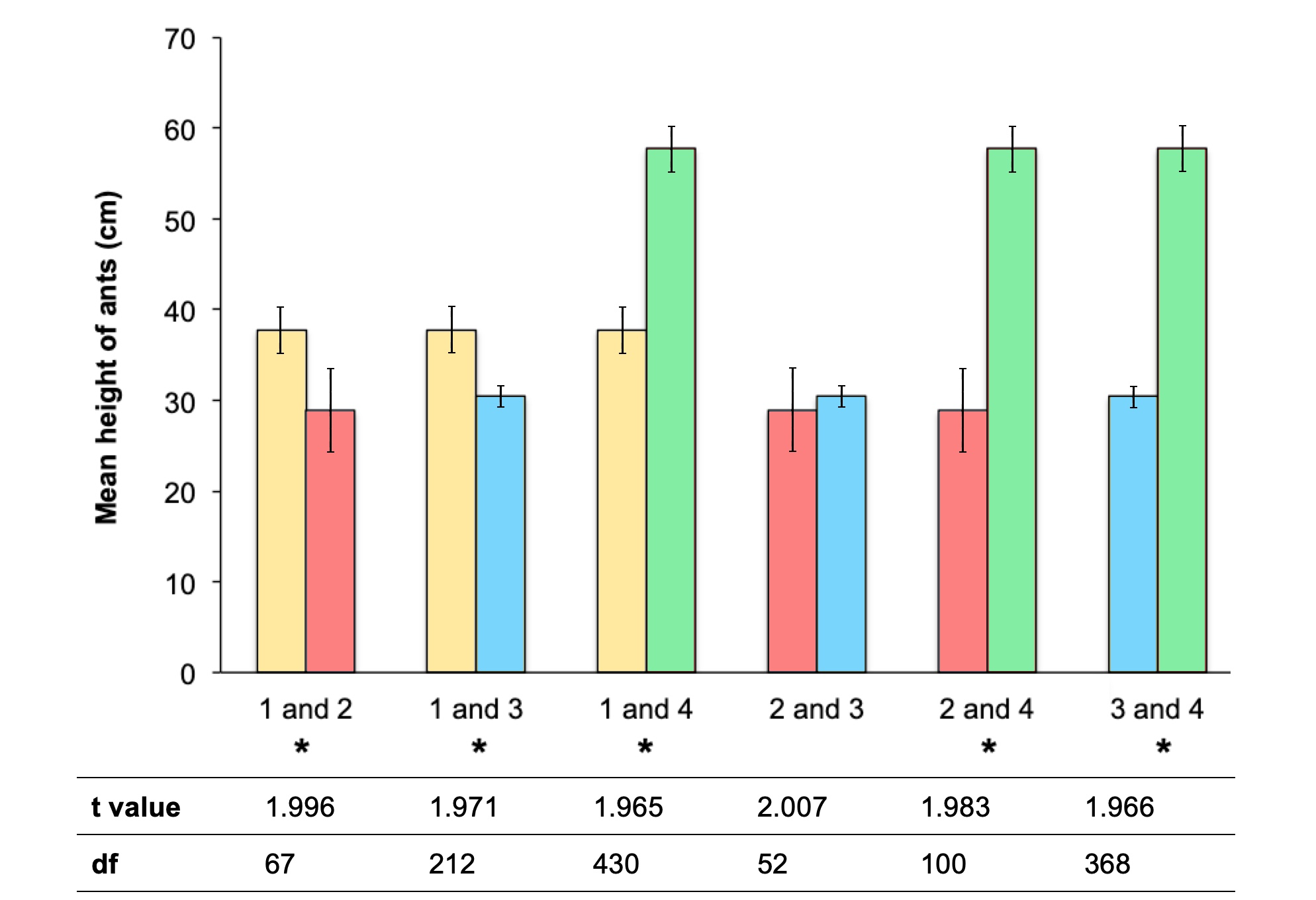
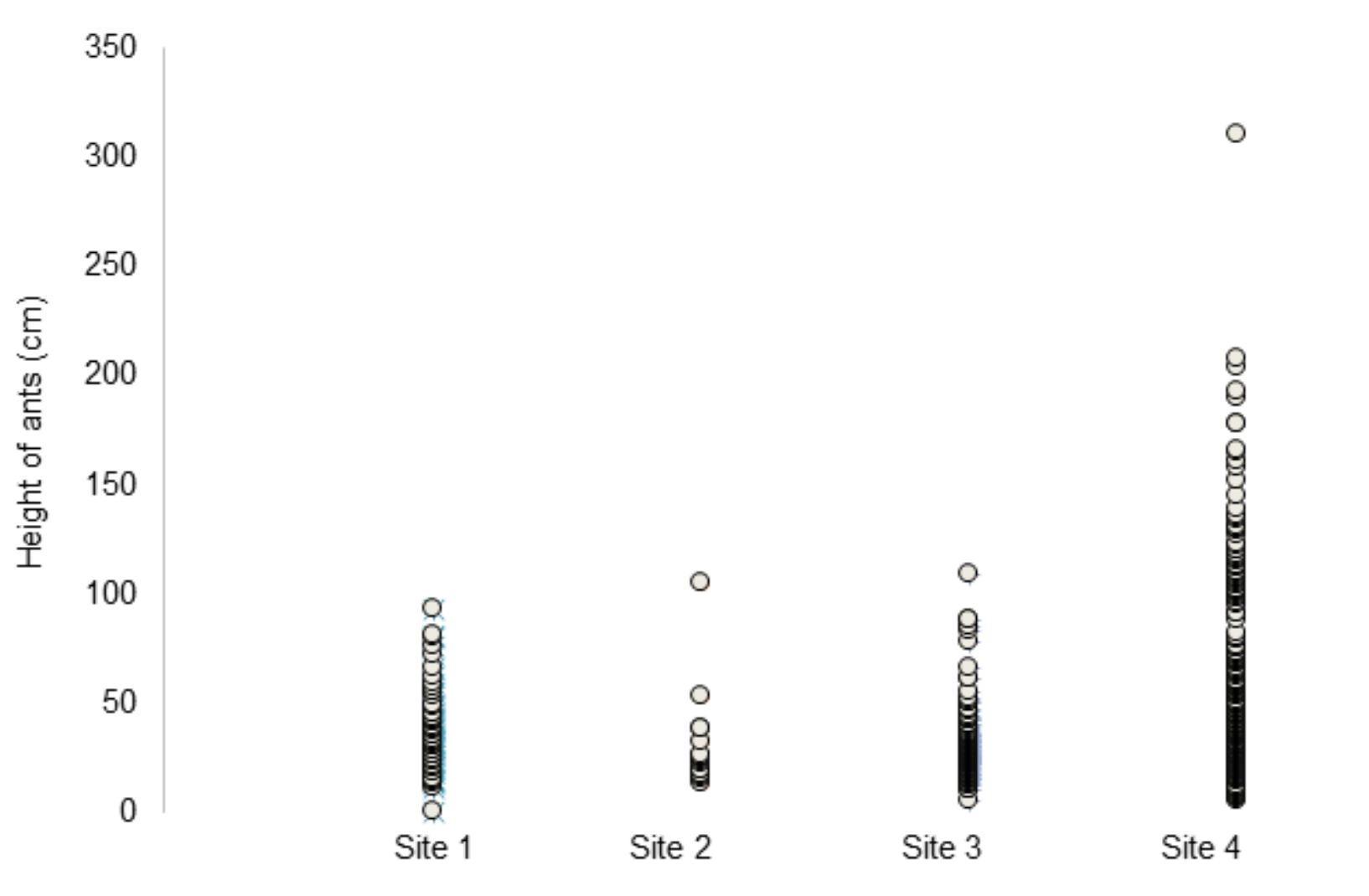
Table 3: Kendall rank correlations for temperature and humidity at Sites 1–4 in Gunung Mulu National Park, Borneo. Significant level set at p < 0.05.
|
Kendall rank correlation |
τ |
p |
|---|---|---|
|
Height vs. Day temperature |
-0.184 |
<0.01 |
|
Height vs. Night temperature |
-0.207 |
<0.01 |
|
Height vs. Day humidity |
0.279 |
<0.01 |
|
Height vs. Night humidity |
-0.155 |
<0.01 |
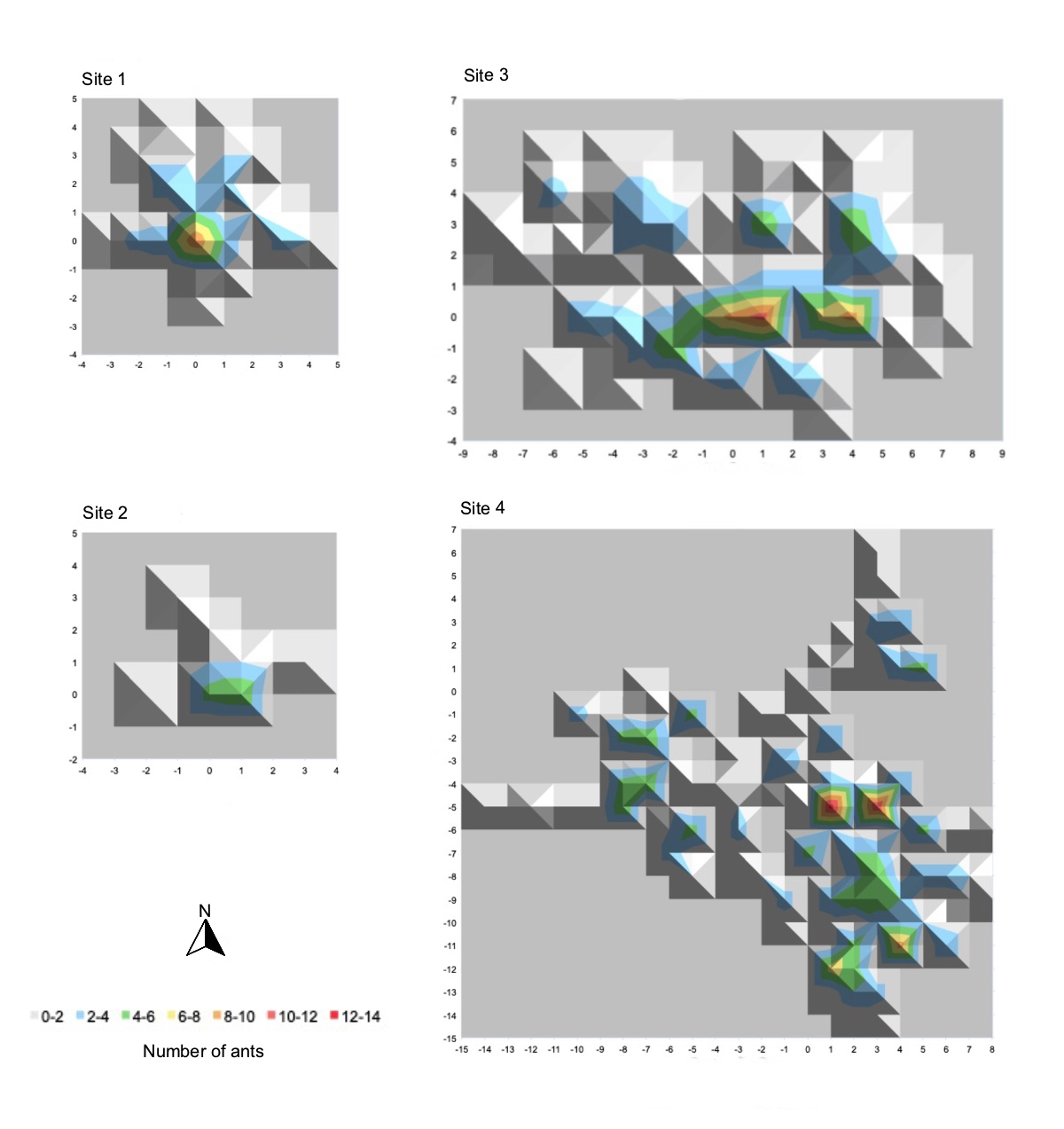
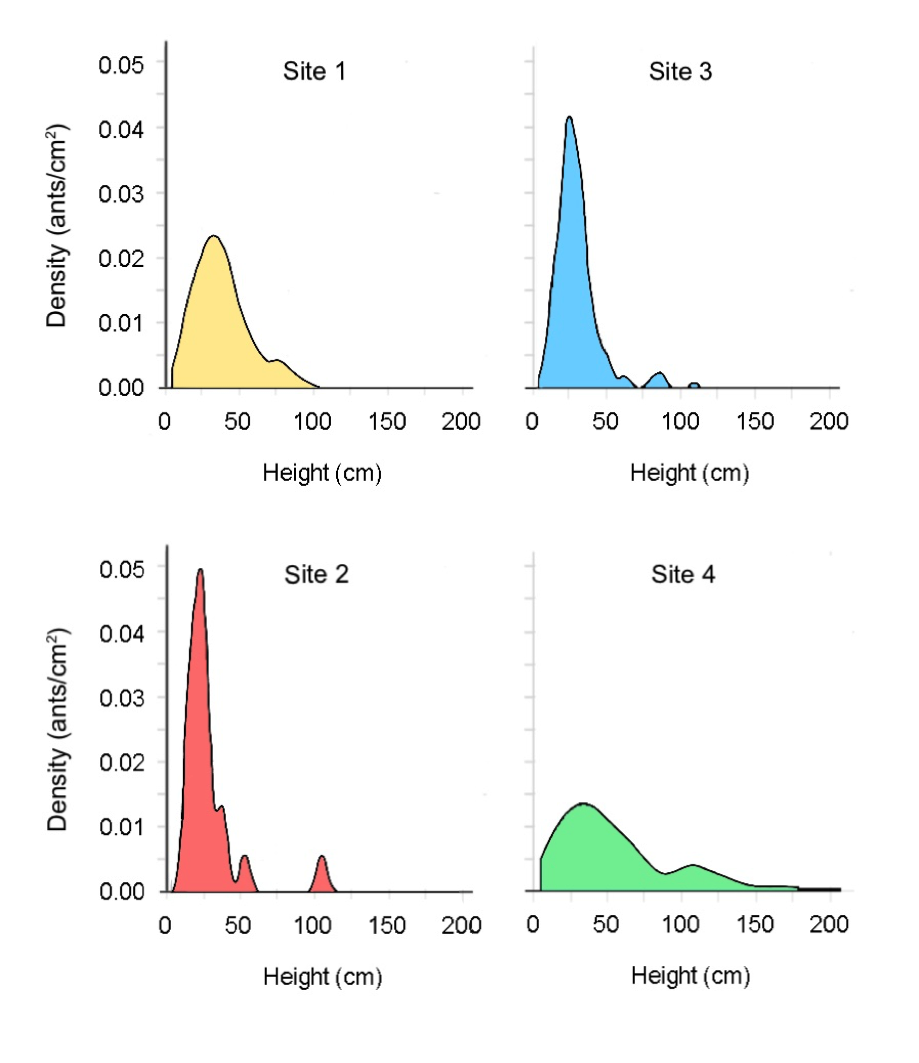
Density maps of the graveyards revealed a patchy distribution of Camponotus leonardi ants infected with Ophiocordyceps unilateralis fungi, with small areas containing high numbers of infected ants surrounded by areas with comparatively few numbers. The average heights of zombie ants in each site were consistently higher than that found by Andersen et al. (2009) (25.20 ±2.46cm), although there was a broad range in heights, with one ant found at ground level and three ants observed at a height of over 2m. The averages in Sites 1, 2 and 4 were also higher than the upper limit of the 95 per cent confidence interval of 20.38 to 30.02cm (Andersen et al., 2009). Only Sites 1 and 4 had median values above 30cm, with a peak density at ~30cm. This can be contrasted to Sites 2 and 3, which had a peak ant density at ~25cm. This indicates that the optimal height of C. leonardi zombie ants in Gunung Mulu National Park may be closer to 25–30cm than the calculated mean values due to inflation from upper values. Potentially, the graveyards at Gunung Mulu had a greater vertical distribution than the sites in Thailand, therefore driving the mean up comparatively. Otherwise, it may be possible that environmental conditions in the tropical forest in Gunung Mulu and Thailand were different – for example, a greater humidity in Gunung Mulu – resulting in a shift in the optimum height at which zombie ants die. This suggests that there is not one optimum height across all environments per se, but an optimal micro-climate that varies in height according to the conditions of a specific environment.
Site area appeared to be positively associated with the maximum height at which zombie ants were found. This potentially results from chance due to higher total numbers of ants present. Most likely, this association is a result of variation in the existing environmental conditions at each site. Two measurable factors clearly distinguished Site 4 from the other sites: its distant location in relation to the other sites, and its distance from the nearest water body. Distance enhances the likelihood of variable conditions in comparison to the other sites due to the effects of natural geographical variation. The strong correlation between distance from water and average height of zombie ants is particularly interesting as this has been previously unexplored. It is possible that proximity to water, and thus a source of evaporation, could be important within a tropical forest as it affects the vertical and horizontal humidity gradients, particularly in the wake of frequent rain and air movement.
As can be seen in Figure 6, the distribution of infected ants appears to be patchy and irregular, with occasional clusters of high ant density. Pontoppidan et al. (2009: 3) obtained similar results in Southern Thailand, observing densities of up to 26 ants/m2 in areas just metres away from quadrats with no infected ants. The maximum observed density in the present study was comparatively lower, at 13 ants/m2; however, the distribution appeared similar, with these high-density regions occurring adjacent to low-density regions. This may suggest that zombie ant graveyards are somewhat dynamic, with the areas of highest infection shifting frequently. Pontoppidan et al. (2009: 3–5) observed that, over time, many of the high-density areas became low density, while conversely, many low-density areas became high density, supporting their hypothesis of a spatially dynamic graveyard. To further explore this hypothesis, it may be beneficial to reassess the same locations at a later date to allow for the analysis of graveyards over time. Andersen et al. (2009: 429) have noted that the spores of the O. unilateralis are too heavy for effective wind dispersal, and thus releasing spores from a greater height is not reproductively beneficial. Instead, spores are actively released to the area immediately below the infected ant, which creates a small but highly infectious region (Andersen et al., 2009). This may be a key factor driving the clumped distribution commonly described, whereby small, highly infectious areas promote the accumulation of deceased and dying infected ants. While this study considered two successive empty quadrats as an edge to the ant graveyard, future researchers may elect to redefine this. By sampling a wider area – for example, continuing until five successive quadrats are empty – it may be observed that graveyard sites eventually overlap. If this were the case, it could be possible that the entire forest or a large area of the forest may correspond to a single extended graveyard, with distinct hotspots of high zombie-ant density. The understanding of the graveyard dynamics across a larger area, as well as assessing the graveyards over time, may provide further insight into the mechanisms of host manipulation and subsequent dispersal success of Ophiocordyceps fungi.
Andersen et al. (2009: 428) noted that when deceased ants were relocated, successful spore dispersal was drastically reduced. It is possible that what appears to be behavioural manipulation is in fact environmental selection of viable graveyards, whereby the habitat selection of ants is in fact random and unsuccessful dispersal simply fails to result in graveyard formation. Due to the tendency of infected ants to lock their jaw irreversibly on the underside of the leaves on which they die (Evans and Samson, 1982: 432), cadavers occurring in micro-environments that are unsuitable to spore dispersal would likely still be observable with a thorough search of the environment. As the graveyards we identified were primarily via a survey of leaves directly adjacent to the path, it is possible that solitary cadavers were missed, and it is suggested that future studies survey habitats systematically prior to the identification of graveyards. However, the primary focus of this study is the vertical spatial distribution of ants within the graveyard, which does show a trend in the occurrence of cadavers within a particular height range. This suggests that there is a mechanism of manipulation that is encouraging ants to be positioned optimally within the vertical gradient.
The four sites had a weak but significant association of temperature and humidity on the mean height of infected ants. This suggests that micro-climate factors, including temperature and humidity, could have some influence on the height of ant cadavers – although other factors may also have an effect, such as vegetation structure. This also indicates that height may not be incidental to cadaver selection and that environmental conditions could instead be intimately related to the spatial dynamics of ant graveyards. However, these measurements were not replicated throughout the study so do not account for any fluctuations present throughout the study period. Measuring temperature and humidity over a longer period may strengthen the validity of these results in the future.
Mean humidity was approximately 90 per cent across sites, which is slightly lower than the optimum level suggested by Andersen et al. (2009) of 94 to 95 per cent. However, the humidity and temperature measurements in this study were taken at standing height at ~1.5m. The study may have benefitted from these measurements being recorded at multiple points on a vertical gradient, where variations may have been more obvious due to vertical vegetation structure. Colonies of C. leonardi nest within the canopy where abiotic conditions are highly variable. In contrast, zombie ants are located close to the ground where temperature is consistently low and humidity consistently high. This illustrates the integral role that stable optimal conditions play in the growth of entomopathogenic fungal spores, whereby fluctuations in humidity and temperature may negatively impact spore development (Andersen et al., 2009; Oduor et al., 1996; Arthurs and Thomas, 2001). Sites 1 and 4, which had the highest relative humidity at standing height, also had greater average heights of zombie-ant occurrence and vertical distributions compared to Sites 2 and 3. It is suggested that high relative humidity (around 94–95 per cent) may result in denser and more widely dispersed zombie graveyards. Dipterocarp forests tend to be an integration of multiple vegetation types and structures; Site 4 had a relatively sparse understory and a tall, dense canopy, particularly compared to Sites 2 and 3, which had dense understories with greater light exposure. Tall, dense canopies can trap moisture released from evaporation and transpiration within the understory, creating a micro-climate of increased humidity (Parker, 1995). This may explain why there was a particularly tall vertical distribution of zombie ants in Site 4, as O. unilateralis would have been better supported by the higher vertical humidity levels.
Interestingly, we observed only two live infected ants within the graveyards, both of which occurred at Site 4. These ants had bitten into vegetation and appeared close to death. As the presence of the graveyard suggested recent spore dispersal and infection of ants within the vicinity, it was expected that more live infected ants would be found displaying signs of infection such as random directionless movement or body convulsions (Hughes et al., 2011). Andersen et al. (2009: 428) and Pontoppidan et al. (2009: 1) noted that C. leonardi typically nest high within the canopy, well above this study’s upper sample limit of 2m. This may have contributed to the reduced numbers of live ants observed, particularly those in the early stages of infection. Pontoppidan et al. (2009: 1) reported that infected ants spent minimal time below the canopy, which suggests that ants in the early stages of infection may have still resided within the canopy at the sites surveyed within this study. It is also possible that the convulsions, known to result in infected ants falling from the canopy (Hughes et al., 2011: 6), occur relatively soon before they ascend into the vegetation and perform the ‘death grip’. This lack of forest floor activity may be evidence of a defensive mechanism, whereby C. leonardi spend most of their time in the canopy to reduce the risk of infection. It may be beneficial to conduct a canopy search to locate live ant nests and current foraging trails to further explore the graveyard spatial dynamics. This could include live ant colonies that may be susceptible to infection in the near future.
The horizontal distribution of infected ants was found to be patchy within graveyard sites. The mean height of infected ants varied between sites, ranging from 28.9 in Site 3 to 57.6 at Site 4. Across all sites combined, 75 per cent of ants were found below 55cm. Our findings suggest that these heights correspond to specific zones of temperature and humidity that optimise spore dispersal and fungal growth, corresponding to the highest levels of subsequent fungal infection. These findings suggest that the behavioural manipulation of O. unilateralis is both intricate and possibly of great importance to the fungi’s success as a parasite. It may be beneficial to further explore the dynamics of zombie-ant graveyards by examining graveyards at a greater horizontal and vertical scale, as well as investigating graveyards over a period of time. It should also be considered that the monitoring of forest areas near existing graveyards could give insight into the emergence of new graveyards and how they develop spatially and temporally. This may expand our understanding of zombie-ant graveyards and potentially reveal how the location, size and density of ant graveyards shift over time, shedding further light on the dynamics of rainforest ecosystems as a whole. It would also aid in better defining zombie-ant graveyards in terms of distinguishing environmental conditions and ant densities.
This research was conducted while studying the unit ‘Tropical Terrestrial Biology’ at Monash University. We are grateful to Monash University for providing us with the opportunity to conduct this research. We wish to express our gratitude to Zoe, Yek Sze Huei, for her guidance and expertise in ant-related fieldwork and assistance in exploring potential research directions. We would also like to thank Gunung Mulu National Park management for permitting fieldwork to take place within the park. Lastly, we would like to thank Jason Gan, Tanneswarry Sethu Pathy, Bethany Xerri, Alanis Olesch-byrne, Jennifer McFee, Ngan Vo and Nicolas Reilly for their help in conducting the fieldwork.
Figure 1: Photograph of infected ant. Photo author’s own.
Figure 2: Map depicting location of sites.
Figure 3: T-test comparing sites in terms of mean ant height.
Figure 4: Height of infected ants by site.
Figure 5: Relationship between height above ground of ants and distance from the nearest water body.
Figure 6: Site density maps illustrating the number of infected ants found per 1m2 quadrat.
Figure 7: Density plots of infected ants at different heights at the four sites.
Table 1: Summary of environmental conditions.
Table 2: Summary of graveyard characteristics at the four sites.
Table 3: Spearman and Kendall rank correlations for temperature and humidity.
Andersen, S. B., S. Gerritsma, K. M. Yusah, D. Mayntz, N. L. Hywel-Jones, J. Billen, J. J. Boomsma and D. P. Hughes (2009), ‘The life of a dead ant: The expression of an adaptive extended phenotype’, The American Naturalist, 174 (3), 424–33
Andersen, S. and D. A. Hughes (2012), ‘Host specificity of parasite manipulation’, Communicative & Integrative Biology, 5 (2), 163–65, available at https://doi.org/10.4161/cib.18712, accessed 10 August 2020
Araújo, J. P. M., H. C. Evans, R. Kepler and D. P. Hughes (2018), ‘Zombie-ant fungi across continents: 15 new species and new combinations within Ophiocordyceps I. Myrmecophilous hirsutelloid species’, Studies in Mycology, 90, 119–60, available at https://doi.org/10.1016/j.simyco.2017.12.002, accessed 10 August 2020
Arthurs, S. and M. B. Thomas (2001), ‘Effects of temperature and relative humidity on sporulation of Metarhizium anisopliae var. acridum in mycosed cadavers of Schistocerca gregaria’, Journal of Invertebrate Pathology, 78, 59–65, available at http://www.thethomaslab.net/uploads/arthurs2001b.pdf, accessed 10 August 2020
Baccaro, F.B. and G. Ferraz (2013), ‘Estimating density of ant nests using distance sampling’, Insectes Sociaux, 60 (10), 103–10, available at https://link.springer.com/content/pdf/10.1007/s00040-012-0274-2.pdf, accessed 10 August 2020
Brown, J. H. (2014), ‘Why are there so many species in the tropics?’, Journal of Biogeography, 41 (1), 8–22, available at https://onlinelibrary.wiley.com/doi/pdf/10.1111/jbi.12228, accessed 10 August 2020
Burchill, A. T. and C. S. Moreau (2016), ‘Colony size evolution in ants: Macroevolutionary trends’, Insectes Sociaux, 63 (2), 291–98, available at https://link.springer.com/content/pdf/10.1007/s00040-016-0465-3.pdf, accessed 10 August 2020
Dawkins, R. (1982), The Extended Phenotype: The gene as the unit of selection, Oxford: Oxford University Press, available at https://web.natur.cuni.cz/filosof/markos/Publikace/Dawkins%20extended.pdf, accessed 10 August 2020
de Bekker, C., L. E. Quevillon, P. B. Smith, K. R. Fleming, D. Ghosh, A. D. Patterson and D. P. Hughes (2014), ‘Species-specific ant brain manipulation by a specialized fungal parasite’, BMC Evolutionary Biology, 14 (166), available at https://doi.org/10.1186/s12862-014-0166-3, accessed 7 April 2020
Evans, H. C. (1974), ‘Natural control of arthropods with special reference to ants (Formicidae) by fungi in the tropical high forest of Ghana’, The Journal of Applied Ecology, 11 (1), 37–49, available at https://www.jstor.org/stable/2402003, accessed 10 August 2020
Evans, H. C., S. L. Elliot and D. P. Hughes (2011), ‘Ophiocordyceps unilateralis: A keystone species for unraveling ecosystem functioning and biodiversity of fungi in tropical forests?’, Communicative & Integrative Biology, 4 (5), 598–602, available at https://doi.org/10.4161/cib.16721, accessed 10 August 2020
Evans, H. C. and R. A. Samson (1982), ‘Cordyceps species and their anamorphs pathogenic on ants (Formicidae) in tropical forest ecosystems I. The Cephalotes (Myrmicinae) complex’, Transactions of the British Mycological Society, 79 (3), 431–53, available at https://doi.org/10.1016/S0007-1536(82)80037-5, accessed 10 August 2020
Fittkau, E. J. and H. Klinge (1973), ‘On biomass and trophic structure of the central Amazonian rain forest ecosystem’, Biotropica, 5 (1), 2–14, available at http://www.jstor.com/stable/2989676, accessed 10 August 2020
Hughes, D. P., S. B. Andersen, N. L. Hywel-Jones, W. Himaman, J. Billen and J. J. Boomsma (2011), ‘Behavioral mechanisms and morphological symptoms of zombie ants dying from fungal infection’, BMC Ecology, 11 (13), available at https://doi.org/10.1186/1472-6785-11-13, accessed 7 April 2020
Jablonski, D., K. Roy and J. W. Valentine (2006), ‘Out of the tropics: Evolutionary dynamics of the latitudinal diversity gradient’, Science, 314 (5796), 102–06, available at http://www.jstor.com/stable/20031477, accessed 10 August 2020
Lefevre, T., S. A. Adamo, D. G. Biron, D. Misse, D. Hughes and F. Thomas (2009), ‘Invasion of the body snatchers: The diversity and evolution of manipulative strategies in host-parasite interactions’, Advances in Parasitology, 68, 45–83, available at https://doi.org/10.1016/S0065-308X(08)00603-9, accessed 10 August 2020
Loreto, R. G., J. P, M. Araújo, R. M. Kepler, K. R. Fleming, C. S. Moreau and D. P. Hughes (2018), ‘Evidence for convergent evolution of host parasitic manipulation in response to environmental conditions’, Evolution, 72 (10), 2144–55
Martín-Vega, D., A. Garbout, F. Ahmed, M. Wicklein, C. P. Goater, D. D. Colwell and M. J. Hall (2018), ‘3D virtual histology at the host/parasite interface: Visualisation of the master manipulator, Dicrocoelium dendriticum, in the brain of its ant host’, Scientific Reports, 8 (8587), available at https://doi.org/10.1038/s41598-018-26977-2, accessed 7 April 2020
Navrátil, M., V. Špunda, I. Marková and D. Janouš (2007), ‘Spectral composition of photosynthetically active radiation penetrating into a Norway spruce canopy: The opposite dynamics of the blue/red spectral ratio during clear and overcast days’, Trees, 21 (3), 311–20
Oduor, G. I., G. J. de Moraes, L. P. S. van der Geest and J. S. Yaninek (1996), ‘Production and germination of primary conidia of Neozygites floridana (Zygomycetes: Entomophthorales) under constant temperatures, humidities, and photoperiods’, Journal of Invertebrate Pathology, 68 (3), 213–22, available at https://doi.org/10.1006/jipa.1996.0088, accessed 10 August 2020
Parker, G. G. (1995), ‘Structure and microclimate of forest canopies’, in Lowman, M. D. and N. M. Nadkarni (eds), Forest Canopies, San Diego: Academic Press, pp. 73–106
Phillips, O. L., P. Hall, A. H. Gentry, S. A. Sawyer and R. Vasquez (1994), ‘Dynamics and species richness of tropical rain forests’, Proceedings of the National Academy of Sciences, 91 (7), 2805–09, available at https://doi.org/10.1073/pnas.91.7.2805, accessed 10 August 2020
Pontoppidan, M. B., W. Himaman, N. L. Hywel-Jones, J. J. Boomsma and D. P. Hughes (2009), ‘Graveyards on the move: The spatio-temporal distribution of dead Ophiocordyceps-infected ants’, PLoS One, 4 (3), e4835, available at https://doi.org/10.1371/journal.pone.0004835, accessed 7 April 2020
Poulin, R. (2000), ‘Manipulation of host behaviour by parasites: A weakening paradigm?’, Proceedings of the Royal Society B: Biological Sciences, 267 (1445), 787–92, available at https://doi.org/10.1098/rspb.2000.1072, accessed 10 August 2020
Sobczak, J. F., L. F. A. Costa, J. L. V. R. Carvalho, G. Salgado-Neto, J. C. M. S. Moura-Sobczak and Y. F. Messas (2017), ‘The zombie ants parasitized by the fungi Ophiocordyceps camponotiatricipis (Hypocreales: Ophiocordycipitaceae): New occurrence and natural history’, Mycosphere, 8 (9), 1261–66, available at http://mycosphere.org/pdf/Mycosphere_8_9_1.pdf, accessed 10 August 2020
Thomas, F., S. Adamo and J. Moore (2005), ‘Parasitic manipulation: Where are we and where should we go?’, Behavioural Processes, 68 (3), 185–99, available at https://doi.org/10.1016/j.beproc.2004.06.010, accessed 10 August 2020
Torres, C. W. and N. D. Tsutsui (2016), ‘The effect of social parasitism by Polyergus breviceps on the nestmate recognition system of its host, Formica altipetens’, PLoS One, 11 (2), e0147498, available at https://doi.org/10.1371/journal.pone.0147498, accessed 7 April 2020
To cite this paper please use the following details: Lavery, M.N., Murphy, C.F.H. & Bowman, E.K. (2021), 'Zombie-ant graveyard dynamics in Gunung Mulu National Park ', Reinvention: an International Journal of Undergraduate Research, Volume 14, Issue 1, https://reinventionjournal.org/article/view/704. Date accessed [insert date]. If you cite this article or use it in any teaching or other related activities please let us know by e-mailing us at Reinventionjournal@warwick.ac.uk.