Hannah Maudsley, University Centre Reaseheath
This study determined if a relationship between observed behaviours and lunar phases was present in captive Cape Porcupines (Hystrix africaeaustralis), a nocturnal species found in UK collections. Previous research on wild populations of hystrix species found that the amount of moonlight a lunar phase presents can limit activity levels. Field studies found that the correlation can be an evolved predatory avoidance tactic in response to moonlight exposure. A captive study aids in comprehending diel cycles and the factors which influence them, as behaviours are not limited to high moonlight phases. Between November 2022 and March 2023, recordings of nocturnal behaviours on dates surrounding distinct lunar phases took place at Reaseheath Mini Zoo. An ethogram based on previous behaviour studies identified behaviours. Data was then analysed using General Linear Regression (GLR). The study aimed to discover if a higher frequency of behaviours would correlate with lunar phases displaying higher levels of moonlight. Although GLMs proved insignificant, the data showed the rate of maintenance and resting behaviours increased during full moons, behaviours associated with predatory avoidance. Findings can provide information on lunar-behaviour relationships in captivity to benefit species-specific care and animal welfare by facilitating environmental challenge, competency and agency or alleviating moonlight-induced stressors to benefit affective welfare states.
Keywords: Hystrix afrucaeaustralis, Temporal, Lunar, Behavioural
Nocturnality is present in most extant mammals and is perceived to be an ancestral trait originating from therapsids in the Triassic period (Angielczyk and Schmitz, 2014; Lovegrove, 2019). The most common theory for mammalian nocturnal evolution is the ‘nocturnal bottleneck hypothesis’, which describes how early mammalians used nocturnality and developed corresponding traits in response to predatory threats from diurnal reptiles (Gerkema et al., 2013; Hall et al., 2012). The main argument supporting this theory is that fossil evidence indicates endothermy in mammalian ancestors, developed due to the limited nocturnal activity of early reptiles due to their ectothermic nature and reliance on solar activity (Clarke and Pörtner, 2010; Gerkema et al., 2013). After the end-Cretaceous extinction event caused predatory reptilians to go extinct, mammals began to radiate and take back non-nocturnal diel cycles (Gerkema et al., 2013). Research in photopigment evolution found that before early mammals became nocturnal, their ancestors displayed more crepuscular diel cycles, a supportive process for this evolutionary theory (Angielczyk and Schmitz, 2014; Gerkema et al., 2013; Lillegraven et al., 1979). Not requiring high visual sharpness associated with daytime activity, most extant mammals, whether diurnal, nocturnal, crepuscular or cathemeral, have retained nocturnal traits and evolved specialisation in other sensory systems (Hall et al., 2012; Torres and Clarke, 2018). With sensory systems adapted for nightlife, nocturnal animals are affected by different environmental cues than diurnal species (Monterroso et al., 2013).
Environmental cues can alter behavioural rhythms (circadian/circannual), affecting how animals interact and respond to their environment (Alcock and Rubenstein, 2019: Gandia et al., 2023).
An example of an environmental cue that affects nocturnal species is the lunar cycle (Kronfeld-Schor et al., 2013; Palmer et al., 2017). Because the Moon orbits the Earth, light reflected from the Sun to the Moon over 29.53 days creates a cycle of visibly different phases; the lunar cycle (Mayoral et al., 2020). The main lunar phases include the full and new moons and the first and third quarter moons, with the first and third quarters producing an identical amount of moonlight (Mayoral et al., 2020). The lunar cycle can affect the behavioural rhythms of mammalian predators and prey. On nights with higher luminosity, a prey species will limit behaviours that may lead to interactions with predators (Palmer et al., 2017; Alcock and Rubenstein, 2019). Yet, predator species such as African Lions (Panthera leo) have a higher success rate when hunting with little moonlight (Packer et al., 2011). Theories imply that the abiotic environmental cue of the lunar cycle has led to trophic coevolution of behaviours to increase predatory and prey success (Halle, 2000; Packer et al., 2011; Palmer et al., 2017; Rubenstein and Alcock, 2019).
The Cape Porcupine (Hystrix africaeaustralis) is a nocturnal mammal within the family Hystricidae under the order Rodentia (Wilson and Reeder, 2005). The species distribution ranges across southern Africa, inhabiting desert, shrubland and grassland habitats (Roze, 2014; Cassola, 2016; Coppola et al., 2019). A field study on the effects of moonlight and seasonality on the temporal activity of Indian crested porcupines found different levels of moonlight exposure caused variations in behaviour (Alkon and Mitrani, 1988).
Results showed that the porcupines were more active during winter months when moonlight exposure was at its lowest as predation risk was at its lowest (Alkon and Mitrani, 1988).
More recently, another field study on the species found moonlight avoidance across four different study sites and found a significant relationship between the behaviour and the phases of the lunar cycle (Mori et al., 2014). During full moons, activity levels were notably lower than those with less moonlight exposure (Alkon and Mitrani, 1988; Mori et al., 2014). Evidence points to moonlight avoidance by prey species developing in the late Miocene era to avoid predators (Barthelmess, 2006).
There is currently little research regarding what effect the lunar cycle has on activity levels in captive Cape Porcupines. Natural biological rhythms and diel cycles are prone to alteration in captivity due to factors such as artificial light, resource availability, visitor presence, schedules and unnatural weather (Sherwen and Hemsworth, 2019). Given the frequency of porcupines in captivity, research into the day-to-day effects of the lunar cycle on the species on top of these factors could provide a baseline for other nocturnal species and change the care captive collections offer, potentially bettering their welfare state (Rose and Riley, 2019).
The research project investigated what effect the lunar cycle has on the nocturnal behaviour of captive Cape Porcupines. This study could benefit Reaseheath Mini Zoo by providing understanding and reasoning behind specific behaviours seen by their porcupines during the lunar cycle. This knowledge will hopefully allow keepers to offer a better standard of care toward the porcupines, potentially causing a more positive welfare state. As a result, the zoo may want to incorporate shaded areas into the enclosure to encourage natural behaviours and outdoor usage. Hypothetically, this could reduce any unnecessary believed predatory stress to the porcupines. However, the zoo may also want to harness predatory avoidance behaviours to encourage environmental challenge, competency and agency (Clark, 2018).
The hypothesis was that the amount of moonlight displayed at each lunar phase would affect the types of behaviours performed by the porcupines. When there are higher amounts of moonlight (full moon), the porcupines may show a higher frequency of behaviours associated with predator avoidance and stress. When moonlight is at its lowest (new moon), the porcupines may show more feeding and movement behaviours due to the lack of visibility, reducing the motivation to display predator avoidance.
The study observed behaviour overnight, utilising camera traps to capture footage of those displayed (Fleming et al., 2014; O'Connell et al., 2011). The method reduced any impacts a human presence may cause on the study and any potential stressors a physical presence may have on the subjects. Being captive, nocturnal individuals, the porcupines may become distressed due to being unused to human exposure during their routine waking hours. Between November 2022 and March 2023 (to ensure recordings covered two lunar cycles), data collection occurred over three nights surrounding and including the peak of each lunar phase, between 7 p.m. and 7 a.m. Behaviours were recorded at intervals of 30 seconds, applying instantaneous scan sampling (Gilby et al., 2010). Each display of behaviours occurring upon the 30-second intervals was tallied and totalled every night for each behaviour.
Because Reaseheath Mini Zoo was within the University Centre Reaseheath (UCR) campus grounds (Reaseheath, Nantwich, CW5 6DF) and has previously facilitated UCR research projects, it was a practical study site. Given that no facilities within a feasible distance housed Cape Porcupines, the Reaseheath Mini Zoo population was selected. Data collection occurred within their usual indoor and outdoor enclosures at Reaseheath Mini Zoo. The subjects have constant, overnight access to both indoor and outdoor enclosures. The outdoors incorporates a naturalistic design, featuring many logs and natural wooden structures. The indoor features a 20-25°C infrared heat lamp and is the most common resting place of the subjects (Personal Communication, 2022).
The subjects for the study were the three Cape Porcupines at Reaseheath Mini Zoo. Individual physical characteristics provided by ZIMS records and personal communication identified the porcupines. The subjects consisted of subject one, an adult female (14 years old), subject two, an adult female (12 years old), and subject three, a castrated adult male (8 years old). Behaviours were distinguished from a well-referenced ethogram of the researcher’s creation using information from other behavioural studies on the species (see appendix A1) (Coppola and Felicioli, 2021; Giné et al., 2011; Mukherjee et al., 2018; Roze, 2014). To identify each lunar phase, the mobile application (app) MoonX was used (Yarotski, 2022).
The dependent variable, the number of behaviours displayed, was measured continuously. The independent variable, the different lunar cycles, was categorical. Therefore, linear regression tests run on RStudio (Version 1.2.1335) were chosen for data analysis (Horton and Kleinman, 2015; RStudio Team, 2019). This test determined if a relationship between the lunar cycle and the frequency of behaviours was present. Data from each subject underwent individual examination to determine if a relationship occurred. Group data analysis also took place to determine an overall effect and evidence of a relationship. As a linear regression test only uses numerical data, the lunar phases were coded as New Moon = 1, First Quarter = 2, Full Moon = 3, Third Quarter = 4 (Horton and Kleinman, 2015).
The study subjects, the study site, the collection timeframe, the method of identifying/measuring Cape Porcupine behaviours, data intervals and the location of the camera traps were all controlled to ensure the study’s validity and limit any influence.
A Shapiro-Wilk test, separately conducted, was done to determine each distribution, Subject One (P= 0.0221), Subject Two (P= 0.033), and Subject Three (P= 0.0288). The median and interquartile range were chosen as descriptive statistics as the data was non-parametric.
A Shapiro-Wilk test was conducted on the total count of all subject’s data to determine distribution (P= 0.210). The median and interquartile range were chosen as descriptive statistics as the data was non-parametric.
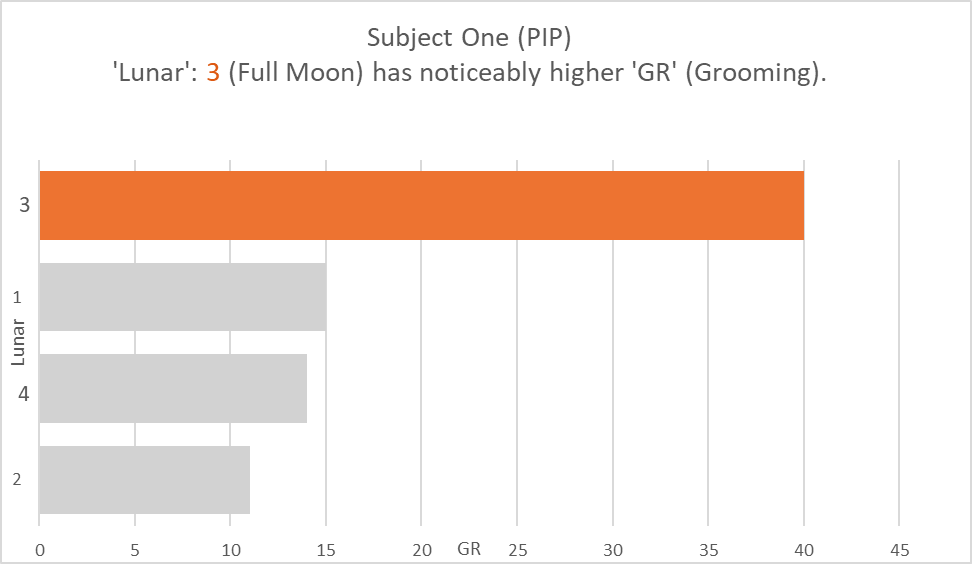
The analysis took place on two months of data on the individual subjects. Evidence of difference was visible from the raw data between the frequency of specific behaviours and specific lunar phases. Graph 1 illustrates that Subject One displayed the grooming (GR) behaviour most frequently on full moons (3) when moonlight was brightest (see Figure 1). Although, as grooming (GR) displays were displayed on new moons (1) more than that displayed on first quarter (2) and third quarter phases (4), the data suggests that luminosity increase did not coincide with the behaviour increase. It instead suggests that behaviour increase not only, occurred when luminosity peaked, but also when it troughed. Graph 2 established a positive association between an increase in luminosity, and the behaviour allo-grooming (AG) displayed by Subject Two increased (See Figure 2). However, as moonlight is the same during first (1) and third quarter (4) phases, the effect on behaviour could have the same effect. Graph 3 indicates how Subject Three demonstrated a high occurrence of the behaviour immobility (IM) on full moons (See Figure 3). The data suggests that for subject three, the increase in immobility (IM) occurred at both maximum and minimum lunar luminosity levels.
General linear models, created using stepwise deletion, determined the most affected behaviours in individual subjects (see Table 1). However, the models found no significant relationship between the lunar phases and the behaviours. The most significant behaviours affected in Subject One were foraging (FG) (Z= 0.643, P= 0.521), socialising (S) (Z= 0.643, P= 0.620) and aggression (AR)= 0.431). The most significant behaviours affected in Subject Two were immobility (IM) (Z= 1.283, P= 0.199), socialising (S) (Z= -1.007, P= 0.314) and flashing (FL) (Z= -0.440, P= 0.660). The most significant behaviours affected in Subject Three were eating (ET) (Z= 706, P= 0.480), aggression (AG) (Z= 0.319, P= 750) and laying down (LD) (Z= 0.483, P= 0.630).

Graph 1. The behaviour GR (Grooming) had a total count of 40 during Lunar 4 (Full Moon) and a count of 15 during Lunar 1 (New moon), indicating an association between the behaviour frequency and the moonlight produced during these cycles in Subject One. The behaviour had a total count of 14 during Lunar 4 (Third Quarter), and 11 during Lunar 2 (First Quarter).
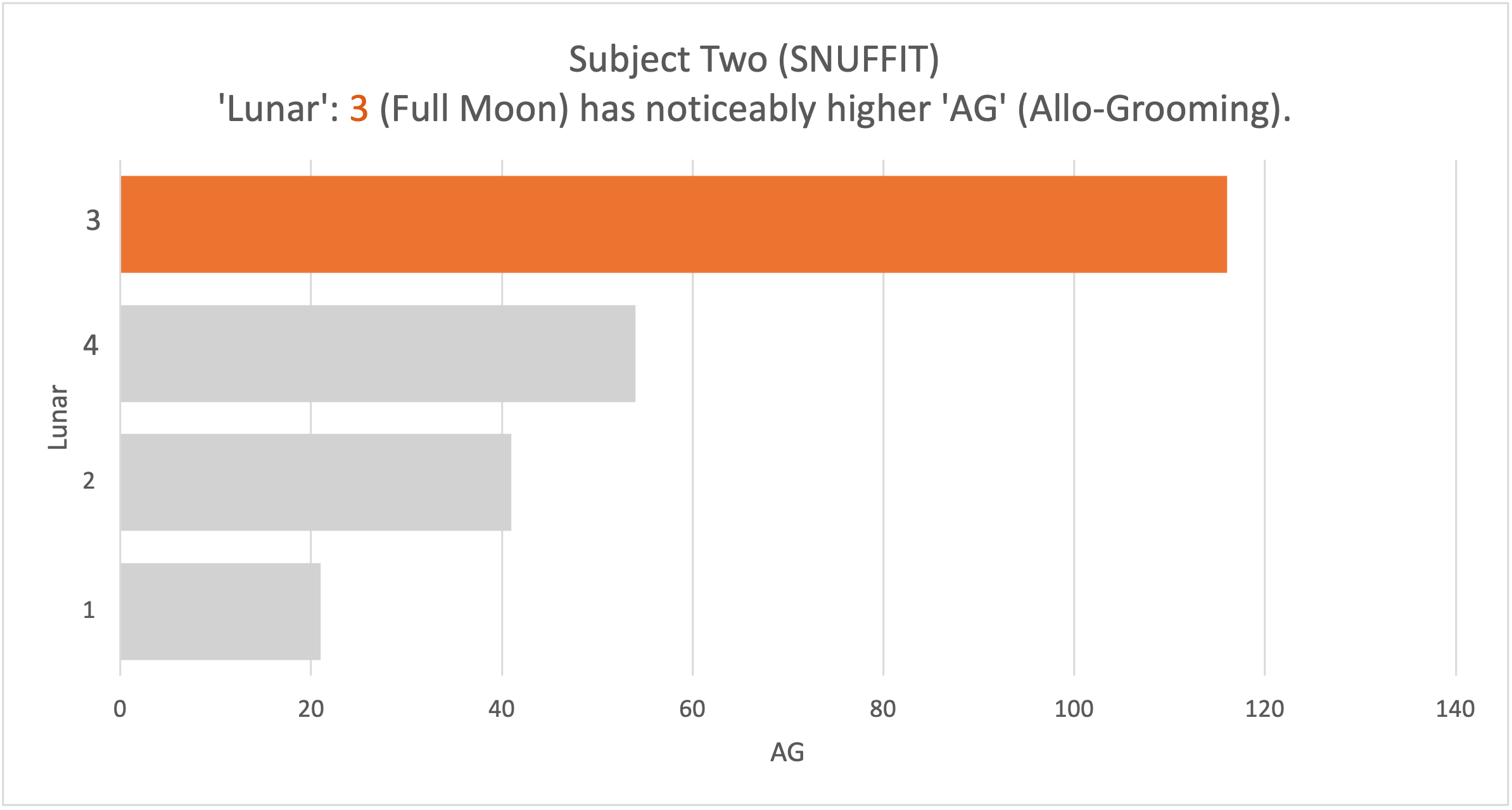
Graph 2. The behaviour AG (allo-grooming) had a total count of 116 during the Lunar 3 (Full Moon). This count reduced as the luminosity of each phase decreased (4 = 54, 1 = 21), indicating a positive association between the frequency of the behaviour and an increase in moonlight in Subject Two.
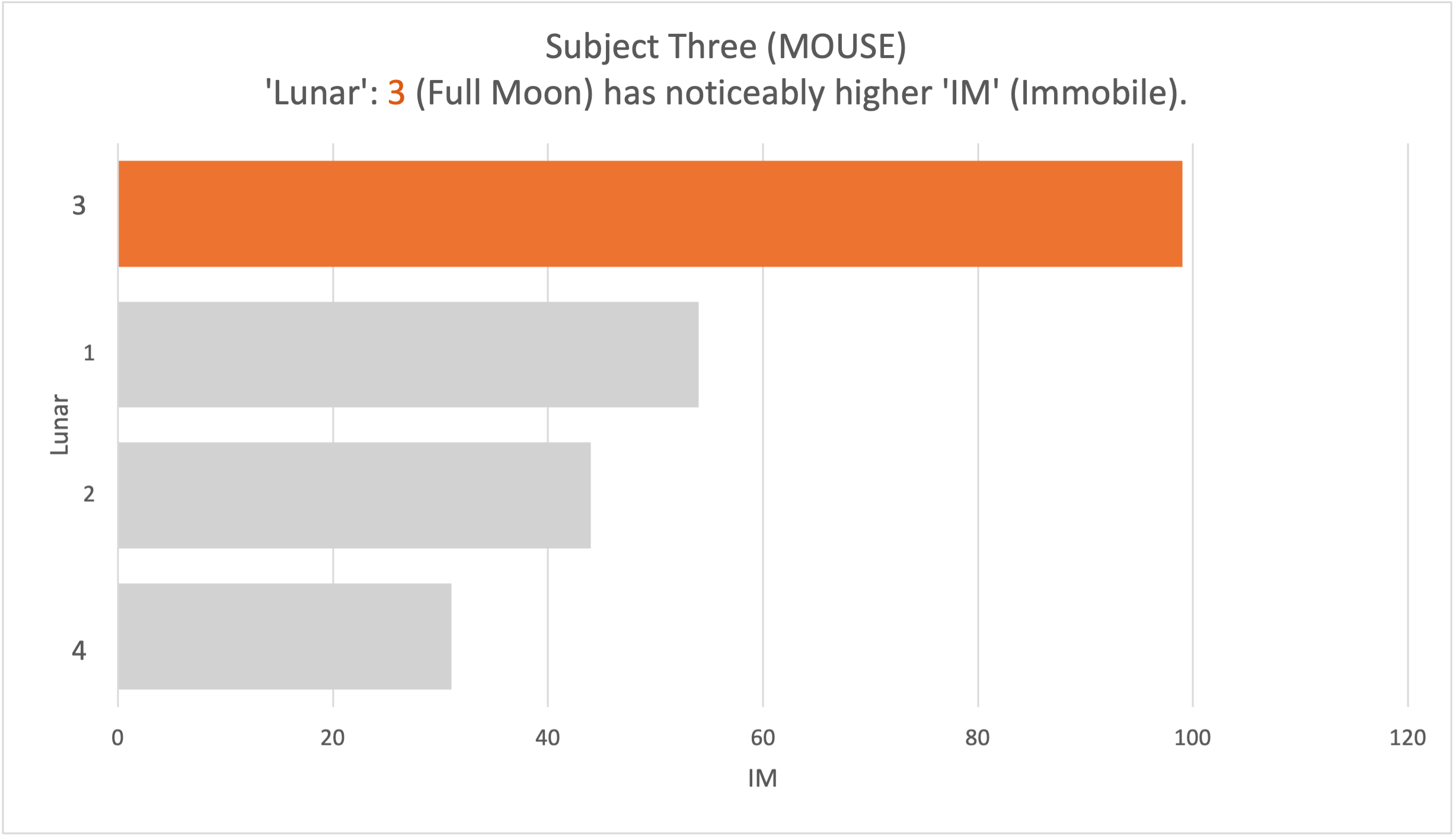
Graph 3. The behaviour IM (Immobile) had a total count of 99 during Lunar 3 (Full Moon) and a count of 54 during Lunar 1 (New Moon), indicating an association between the behaviour frequency and the moonlight produced during these cycles in Subject Three. The behaviour had a total count of 31 during Lunar 4 (Third Quarter), and 44 during Lunar 2 (First Quarter).
| Predictor | Estimate | Standard Error | Z-value | P-value | |
| Subject One (PIP) | Intercept | 1.003 | 0.255 | 3.932 | 8.41e-05 |
| FG | 0.013 | 0.021 | 0.643 | 0.521 | |
| S | -0.010 | 0.020 | -0.496 | 0.620 | |
| AR | -0.103 | 0.126 | -0.818 | 0.431 | |
| Subject Two (SNUFFIT) | Intercept | 0.965 | 0.238 | 4.046 | 5.2e-05 |
| IM | 0.023 | 0.018 | 1.283 | 0.199 | |
| S | -0.037 | 0.037 | -1.007 | 0.314 | |
| FL | -0.025 | 0.056 | -0.440 | 0.660 | |
| Subject Three (MOUSE) | Intercept | 0.778 | 0.230 | 3.370 | 0.001 |
| ET | 0.006 | 0.008 | 0.706 | 0.480 | |
| AG | 0.001 | 0.004 | 0.319 | 0.750 | |
| LD | 0.004 | 0.008 | 0.483 | 0.630 |
The analysis took place on two months of data on the collective sum of all subjects. Evidence of difference was visible from the raw data between the frequency of specific behaviours and specific lunar phases. Graph 4 shows a coinciding increase in the frequency of the behaviour AG as the amount of moonlight increased through the lunar phases (see Figure 4).
A general linear model, created using stepwise deletion, determined the most frequent behaviours displayed by the collective sum of all subjects (see Table 2). However, the model found no significant relationship between the lunar phases and the behaviours. The most significant behaviours affected for the group were socialising (S) (Z= -0.774, P= 0.439), immobility (IM) (Z= 1.033, P= 0.302), and foraging (FG) (Z= 0.350, P= 0.726).
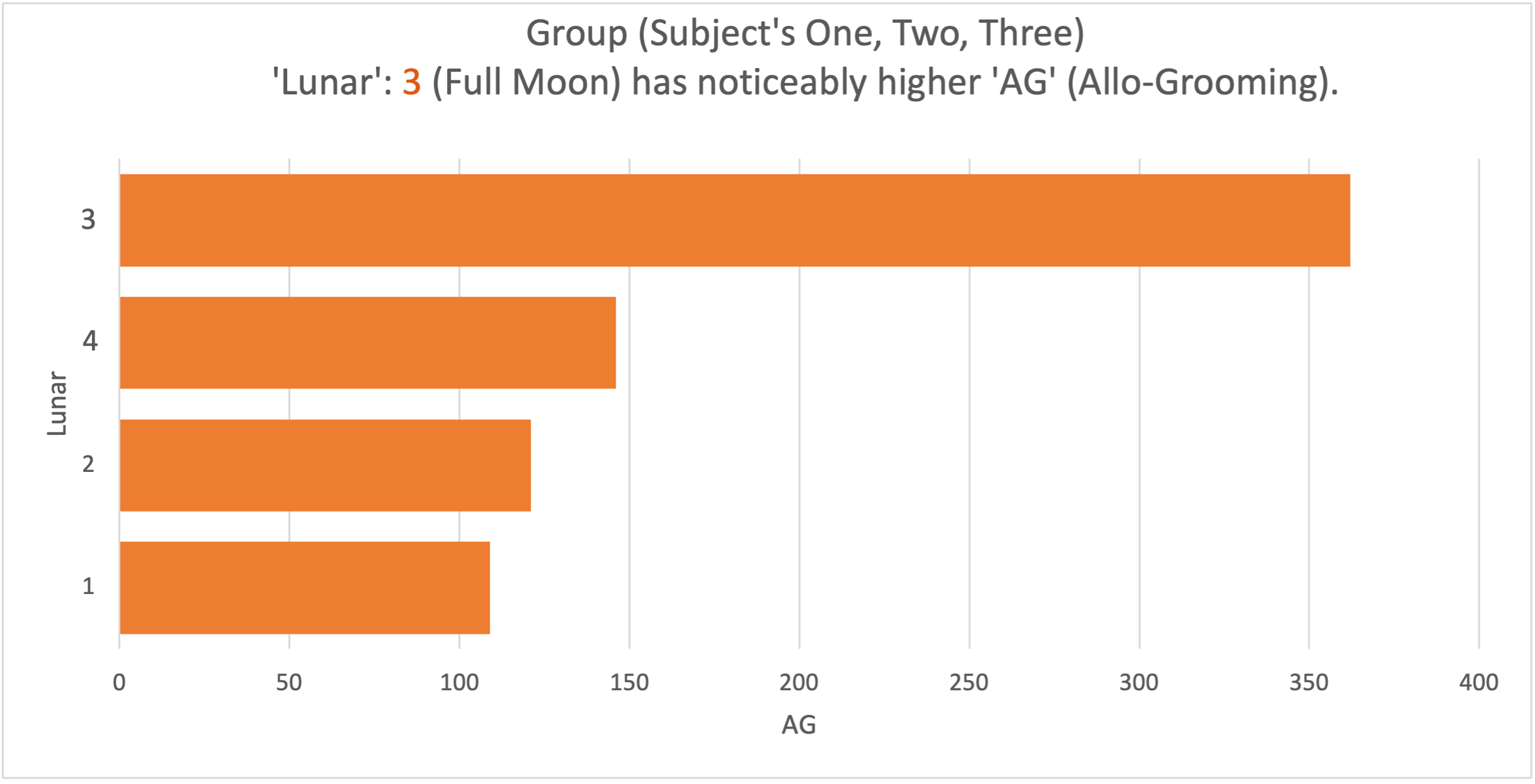
Graph 4. The behaviour AG (allo-grooming) had a total count of 362 during the Lunar 3 (Full Moon). This count reduced as the luminosity of the phases decreased (1 = 109), indicating a positive association between the frequency of the behaviour and an increase in moonlight in all subjects. As Lunar 2 and 4 produce the same luminosity, behaviour may be affected similarly, explaining the lack of variation between the two.
| Coefficient | Estimate | Standard Error | Z-value | P-value | |
| Group (All Subjects) | Intercept | 0.768 | 0.286 | 2.681 | 0.007 |
| S | -0.008 | 0.011 | -0.774 | 0.439 | |
| IM | 0.007 | 0.006 | 1.033 | 0.302 | |
| FG | 0.002 | 0.007 | 0.350 | 0.726 |
A difference in the frequency of specific behaviours regarding particular lunar cycles was evident from the data collected. As moonlight levels intensified across the lunar cycle, an increase in resting and maintenance behaviours was observed. The behaviours grooming (GR) (Subject One), allo-grooming (AG) (Subject Two), and immobility (IM) (Subject Three) were most associated with the full moon lunar phase when moonlight was highest. Similarly, the group showed an association between the behaviour of AG and the full moon. Maintenance behaviours are performed by rodents in an evolved sequence which is prone to alteration by external and internal stimuli such as stressors and neurological differences (Smolinsky et al., 2009). For many mammalian species, maintenance behaviours are perceived as stereotypical in captive animals, indicating a negative welfare state (Smolinsky et al., 2009; Pomerantz et al., 2013). However, contrasting studies suggest that associating high stress with increased grooming behaviours is an oversimplification due to the behaviours’ complexity (Kalueff and Tuohimaa, 2005). Even so, rodents experience increased maintenance behaviours at high or low-stress levels (Kalueff and Tuohimaa, 2004; Moyaho and Valencia, 2002). The two extremes differ as stress-induced grooming occurs in short bursts, at rapid rates, and is often incomplete and interrupted (Kalueff and Tuohimaa, 2005; Smolinsky et al., 2009). Placing this information into context regarding the results, as the subjects are captive individuals, the increase in maintenance behaviours is interpreted as a stereotypical stress response (Kalueff et al., 2016; Smolinsky et al., 2009). However, this does not have to indicate a negative welfare state in the subjects.
The heightened presence of the behaviour IM, a resting behaviour, suggests that these behaviours are an evolved anti-predatory response (Barthelmess, 2006). However, the analysis results suggest no significant relationship between the lunar cycle and the behaviours. The estimates and z-values from the individual GLMs suggest a positive average change in the resting and maintenance behaviours as moonlight increased (see Table 1). The group GLM again implied a positive average change in the behaviours of IM and FG as moonlight increased (see Table 2). These positive changes can also be interpreted as an anti-predatory response to the rising exposure (Barthelmess, 2006). However, the increase in feeding behaviours indicates a captive influence (Sherwen and Hemsworth, 2019). Living in captivity can affect natural rhythms and diel cycles, which could cause the porcupines to unnaturally increase their foraging and eating levels when moonlight is high due to food being available (Berger, 2011; Giné et al., 2011).
The data and interpretations strengthen the hypothesis that the amount of moonlight displayed at each lunar phase would affect the types of behaviours performed by the porcupines. When there are higher amounts of moonlight (full moon), the porcupines may show a higher frequency of behaviours associated with predator avoidance and stress. When moonlight is at its lowest (new moon), the porcupines may show more feeding and movement behaviours due to the lack of visibility, reducing the motivation to display predator avoidance. However, the lack of significance suggested by the GLMs contradicts the hypothesis as they imply the amounts of moonlight had little to no effect on the porcupine’s behaviours. The data from Subjects One and Three suggest that the two extremes of moonlight caused the most frequent behaviour displays.
This point supports the hypothesis due to the types of behaviour, GR and IM, as they are associated with predator avoidance and show moonlight has some effect on the behaviours (Barthelmess, 2006; Kalueff et al., 2016; Smolinsky et al., 2009).However, it does dispute the hypothesis as although an interpreted anti-predatory effect occurs, behaviours are not limited to high moonlight phases.
Considering previous field studies on the effects of moonlight on other hystrix species, the results share similar findings. These studies on the effects of moonlight on porcupines found a significant relationship between moonlight and activity levels, with findings discussing how activity levels were highest when moonlight exposure was reduced (Alkon and Mitrani, 1988, Mori et al., 2014). A study researched the impact of seasonality on activity levels (Alkon and Mitrani, 1988). Regardless of the lunar phase, this study found activity levels were higher during the winter as the weather reduced moonlight exposure (Alkon and Mitrani, 1988). However, other studies found a trigger-like relationship between the lunar phases and porcupine activity budgets, as even when invisible, full moons hindered activity levels and reduced behaviours (Mori et al., 2014). The results from this study uphold the field findings as, although significance was not found, from the GLMs, the increase in maintenance and resting behaviours upon full moons observed from the raw data backs up their hypothesis.
These findings could prove significant by providing a baseline for studies on other nocturnal species. Nocturnal diel cycles exist in most mammalian species, and moonlight can raise predation risk for these species, not limited to the hystrix genera (Prugh and Golden, 2014).
The evolutionary traits and behaviours associated with nocturnal diel cycles can translate into captivity, where artificial factors can cause an unnatural response (Sherwen and Hemsworth, 2019).
Although, field evidence of porcupine species displaying ‘sunbathing’ behaviours in the wild during diurnal hours contradicts this point (Coppola et al., 2019). If exhibited in captivity, arguments would point to the behaviour being a natural physiological response from the porcupine (Coppola et al., 2019).
Studies into the effects of moonlight on prey have previously neglected the impact on foraging efficiency, which has the potential to translate into captive individuals (Prugh and Golden, 2014; Sherwen and Hemsworth, 2019). Foraging was suppressed in rodent species when higher levels of moonlight were present to the point where it reached similar levels to that of a predatory presence (Prugh and Golden, 2014). In captivity, this could cause negative welfare implications encouraging stereotypical behaviours and increased states of stress (Rose and Riley, 2019). As visitors have more enjoyable experiences when animals are present, some studies have recommended using environmental enrichment to encourage diurnal activity in nocturnal species (Fernandez et al., 2009; Margulis et al., 2003). The presence of visitors could be mistaken by nocturnal species as a predatory presence, causing foraging suppression as a predator avoidance reaction, leading to a potential negative welfare state (Fernandez et al., 2009; Rose and Riley, 2019).
As an implication of the results, Reaseheath Mini Zoo may wish to incorporate changes to their husbandry routines and enclosure design. The insight from this study can provide the zoo with context to specific behaviours observed in the group that can justify changes.
The zoo may wish to incorporate shaded areas into the outdoor enclosure to reduce stress-induced maintenance behaviours and better the subject’s welfare (Rose and Riley, 2019; Smolinsky et al., 2009). The porcupines can benefit from this as the reduction in moonlight exposure on the third quarter and whole moon cycles and limiting unnecessary predator avoidance cues (Kalueff and Tuohimaa, 2005; Mori et al., 2014). A potential increase in outdoor enclosure usage may result from these shaded additions, possibly bettering welfare and reducing hostile interactions between con specifics (Nogueira et al., 2004).
Providing shaded areas can also benefit the subjects during daytime hours. As captivity can change biological cycles and circadian rhythms due to husbandry and feeding routines, it is common to see captive porcupines with more cathemeral diel cycles (Corsini et al., 1995; Hagen et al., 2020; Ramírez-Chaves et al., 2020; Sherwen and Hemsworth, 2019). Due to their retained evolutionary traits for nocturnal life, this sunlight exposure can potentially impact health and welfare (Rose and Riley, 2019; Torres and Clarke, 2018). Shaded areas can relieve pressures on their nocturnal traits while not restricting their activity levels and benefit visitor experiences (Fernandez et al., 2009). Limiting husbandry routines to early or late hours can reduce any disturbance caused to the diel cycles of the porcupine and limit interference with important resting behaviours (Gandia, 2023).
However, the biological perception and response to the lunar cycle are not solely related to luminosity levels. Studies hypothesise that because geomagnetic activity fluctuates around specific lunar phases, animals sensitive to this could display changes in behaviour and activity levels in response (Bevington, 2015; Chakraborty, 2020; Nishimura and Fukushima, 2009).
The effects of climate change could affect lunar perception – for example, changes in temperature, humidity and weather that may coincide around lunar phases could lead to variations in the behaviours of nocturnal mammals as a response (Camuffo, 2001; Chakraborty, 2020; Rode-Margono and Nekaris, 2014).
To exemplify, satellite technology confirmed the moon impacts global temperatures, with warmth coinciding with the full moon (Balling Jr and Cerveny, 1995). Behaviour studies indicate that the collaboration between temperature and moonlight influenced the observed nocturnal activity pattern (Fernandez-Duque, 2003).
Reaseheath Mini Zoo may wish to harness predatory avoidance behaviours to encourage environmental challenge, competency and agency (Clark, 2018). Promoting predatory avoidance allows the porcupines to acquire a repertoire of natural cognitive skills they can use when faced with a challenge (Clark, 2017). This promotion provides the porcupines with a more wild-like, enriching experience of captivity that can limit boredom and reduce the capacity for stereotypical behaviour development (Clark, 2017).
To promote predatory avoidance, Reaseheath Mini Zoo could shift the porcupine’s feeding schedule to later hours to limit disturbing diurnal resting behaviours (Savenije et al., 2010). Automatic scatter feeders timed for these hours can be used to achieve this (Andrews and Ha, 2014; Law and Reid, 2010). These devices can increase feeding and moving behaviours by encouraging foraging and increased enclosure usage (Andrews and Ha, 2014). They can also reduce contra-freeloading as the devices are often portable and can be moved to separate locations and set to unpredictable times each night (Clark, 2017).
Opposed to this, the zoo could change feeding times to earlier/later in the day to reduce potential disturbances to the diel cycles and resting behaviours (Savenije et al., 2010).
Utilising camera traps for this study was beneficial as it allowed the collection of a sizeable amount of data and minimised any harm to the researcher and the subjects. Camera traps limited hypothetical stressors that may have become apparent from an unknown physical presence. This method also reduced the collection of influenced data human presence could have caused due to the unfamiliarity of human company at late hours. Overall, this method reduced anthropogenic impacts that may have negatively affected animal welfare or manipulated the data (Caravaggi et al., 2017). The camera traps also covered multiple areas of the enclosure to get the best possible view of the behaviours displayed at the chosen intervals (Caravaggi et al., 2017). The camera traps also reduced human error from missing collection intervals and refined the study because of the pause and replay functions. In-person behaviour observation may have led to a lack of data in the study due to distractions. However, technical problems did occur due to weather and limited power sources.
The camera traps ran on 12 AA alkaline batteries, prone to failure during cold weather (Palacín and de Guibert, 2016). As the collection period fell between November 2022 and March 2023, the weather was often colder and unpredictable. A solution to this would be an alternative power source like Lithium-Ion batteries that fare better in colder environments (Dagger et al., 2017).
Significance in the relationship between the lunar cycles and nocturnal porcupine behaviours had a higher probability of being found from the GLMs if the data sets were larger (Bakeman and Quera, 2012).
Increasing data collection time frames, covering more lunar cycles, collecting data from multiple collections, utilising more camera traps to capture more angles and limiting the frequency of invisible behaviours recorded could correct this issue. Conducting the study over multiple seasons, particularly over summer, could provide a better range of data because extreme weather such as rain and snow can hinder porcupine activity and increase resting behaviours from limited outdoor exposure (Alkon and Mitrani, 1988).
Given the frequency of porcupine species in UK zoos, the findings of this study can provide insight into how alterations to husbandry and animal care can impact the effect of lunar cycles. Research on the behavioural impacts of lunar cycles on their porcupines can allow Reaseheath Mini Zoo to encourage a better welfare state in their prickle (collective noun for porcupines). By integrating suggestions from this study, collections may increase the probability of their porcupine populations adopting a positive welfare state. However, as it is not unusual for diel cycles to change in captivity, it can be argued that because zoo visitors have a better experience when animals are visible, encouraging a more cathemeral diel cycle in Cape Porcupines should be fostered as is likely to occur otherwise. Conversely, this can change how porcupines respond to environmental cues like the lunar cycle and impact how they interact with their environment. By understanding the findings from this study, collections can adapt captive life for their porcupines to encourage natural cued responses. The take-home message from this study is that behavioural change was visible in the subjects concerning the amount of moonlight produced by the lunar phases. This behavioural change can cause positive and negative welfare implications on the affective state of the porcupines on an individual and group scale. Under the Zoo Licencing Act (1981), UK zoological collections must abide by the provisions stated in the Secretary of State’s Standards of Modern Zoo Practice (2012)- ‘SSSZMP’, the fourth provision being supplying the opportunity for natural behaviours. As the evidence points to predatory avoidance being an evolutionary trait, collections can meet this provision using the findings and suggestions from this study. Promoting natural diel cycles can also be done to meet this provision and ensure that the animal’s welfare requirements meet the standard of the SSSMZP (2012).
Investigation into additional factors that may affect the behaviour and activity budgets of Cape Porcupine would further this study. As data collection methods became impacted by weather, further developments could pair together how different weather affects the behaviours of captive Cape Porcupines regarding the lunar cycle. Studies on the effect of seasonality have touched on the subject concerning wild Indian crested porcupines (Hystrix indica) in the field (Alkon and Mitrani, 1988). Although done on a similar topic and species, development could benefit from moving in a more focused direction regarding the effect of weather and lunar phases.
Analysis of the before and aftereffects and value of incorporating shaded areas into enclosure design on nocturnal mammals in captivity would be a good direction for this study to expand.
From the study, a potential relationship between the lunar cycle and enclosure usage arose. The subjects at Reaseheath Mini Zoo have ad-libitum access to both an indoor and outdoor chamber, where an impact on preference may have been because of moonlight exposure. Based on evidence regarding the porcupine’s anti-predatory responses, it is likely that, on brighter lunar phases, indoor enclosures are preferred (Mori et al., 2014). Development on this can come from the results of this study because the behaviours with the highest growth regarding risen moonlight levels were those of resting and maintenance. These behaviours could have a significant association with the indoor enclosure as this is the favoured resting site of the subjects (Personal Communication, 2022). However, due to the variation in UK weather, it would need to be considered as a potential influence on enclosure preference.
Another question spurred by the research was the effect of the lunar cycle on sex in Cape Porcupines. As the subject did not consist of an equally split group of males and females, with there being two females and one male, who was castrated, this was not a considered factor. However, from the results, Subject Three (male) showed a significant increase in resting behaviours when moonlight was brightest. However, in Subject One (female) and Subject Two (female), a rise was seen in maintenance behaviours. Observations on hystrix reproductive behaviours have previously occurred. However, context and insight focus on wild individuals and habitat effects (Coppola and Felicioli, 2021; Van Aarde, 1987). Further research on this factor can benefit captive breeding programmes as they may find a higher rate of breeding success when lunar cycle activity budgets are understood.
The study lacked finding an overall relationship between lunar cycles and Cape Porcupine behaviours due to the limited sample size. Therefore, further research on the question would provide a clear outlook on the effect on the whole species rather than one collection. The importance of future research for the species is that it can further the interest in finding the relationship in other captive hystrix species, captive rodents or captive nocturnal animals. Collections can then use this information to ensure that welfare provisions in captivity can be specialised to suit the needs of these categories, possibly bettering their welfare states.
| Feeding behaviours | ||
| - Eating | ET | Chewing, swallowing, consuming food item (Mukherjee et al., 2018). |
| - Foraging | FG | Browsing/searching for food items/water (Giné et al., 2011). |
| - Drinking | DR | Consuming water. |
| Resting behaviours | ||
| - Immobile position | IM | Standing still on four limbs for more than 2 seconds. |
| - Laying Down | LD | Laying down on ground but awake. Front limbs extended in front of face and hind limbs bent at side of body (Mukherjee et al., 2018). |
| - Sleeping | SL | Unconscious, eyes closed, no movement laying down on ground (Giné et al., 2011). |
| Moving behaviours | ||
| - Locomotion | LM | Moving around the enclosure at a steady pace with no food involved. Moving all four limbs individually. |
| - Running | RN | Moving around the enclosure at a quick pace with no food involved. Moving all four limbs individually. |
| - Social interaction | S | Two or more con specifics interacting in close contact with one another for more than 2 seconds (Felicioli et al., 1997). |
| - Aggression | AR | Using quills, teeth, body force to physically harm a con-specific or human (Roze, 2014; Mori and Ferrari., 2021). |
| - Digging | DG | Breaking up of dirt using claws and front limbs (Mukherjee et al., 2018). |
| - Object Play | OP | Interaction with an inanimate object for more than 2 seconds, may or may not involve a con-specific (Mukherjee et al., 2018). |
| - Con-specific Play | CP | Interaction with a con-specific e.g., leaping on one another. Performed in a positive manner for longer than 5 seconds (Mukherjee et al., 2018). |
| Maintenance behaviours | - Grooming | GR | Moving claws front to back in a ‘combing’ motion to clean fur. |
| - Allo-grooming | AG | Mutual grooming involving licking the tail, licking the head or the neck of another con-specific (Felicioli et al., 1997). |
| - Urinating/Defecating | UD | Release of urine/faeces. |
| Communication | ||
| - Rattling | RT | Vibrating tails/rattle quills to produce a warning sound. |
| - Flashing Quills/Mane | FL | Flaring quills and raising mane to increase body size (Roze, 2014). |
| - Vocalisations | VO | Creation of sound from individual/s (Roze, 2014). |
| Missing behaviours | ||
| - Invisible | IN | Individuals not visible/behaviour not captured. |
| Other | O | Observed behaviours not listed within this ethogram. |
Alkon, P. U. and D. S.Mitrani (1988), ‘Influence of season and moonlight on temporal-activity patterns of Indian crested porcupines (Hystrix indica)’, Journal of Mammalogy, 69 (1), 71–80, https://doi.org/10.2307/1381749
Alcock, J. and D. R. Rubenstein (2019), Animal Behaviour, Sunderland, Massachusetts: Sinauer Associates.
Andrews, N. L. and J. C. Ha, (2014), ‘The effects of automated scatter feeders on captive grizzly bear activity budgets’, Journal of Applied Animal Welfare Science, 17 (2), 148–56. https://doi.org/10.1080/10888705.2013.856767
Angielczyk, K. D. and L. Schmitz (2014), ‘Nocturnality in synapsids predates the origin of mammals by over 100 million years’, Proceedings of the Royal Society B: Biological Sciences, 281 (1793), 20141642. https://doi.org/10.1098/rspb.2014.1642
Balling Jr, R. C. and R. S. Cerveny (1995), ‘Influence of lunar phase on daily global temperatures’, Science, 267 (5203), 1481–83. https://doi.org/10.1126/science.267.5203.1481
Bakeman, R. and V. Quera (2012), ‘Behavioral observation’, in H. Cooper, P. M. Camic, D. L. Long, A. T. Panter, D. Rindskopf and K. J. Sher (Eds.), APA Handbook of Research Methods in Psychology, Vol. 1. Foundations, Planning, Measures, and Psychometrics (pp. 207–225), Washington: American Psychological Association. https://doi.org/10.1037/13619-013
Barthelmess, E. L. (2006), ‘Hystrix africaeaustralis’, Mammalian Species, 2006 (788), 1–7. https://doi.org/10.1644/788.1
Berger, A. (2011), ‘Activity patterns, chronobiology and the assessment of stress and welfare in zoo and wild animals’, International Zoo Yearbook, 45 (1), 80–90 https://doi.org/10.1111/j.1748-1090.2010.00121.x
Bevington, M. (2015), ‘Lunar biological effects and the magnetosphere’, Pathophysiology, 22 (4), 211–22. https://doi.org/10.1016/j.pathophys.2015.08.005
Camuffo, D. (2001), ‘Lunar influences on climate’, in Earth-Moon Relationships: Proceedings of the Conference held in Padova, Italy at the Accademia Galileiana di Scienze Lettere ed Arti, 8–10 November 2000 (pp. 99–113), Springer Netherlands. https://doi.org/10.1007/978-94-010-0800-6_10
Cassola, F. (2016), ‘Hystrix africaeaustralis (errata version published in 2017)’, The IUCN Red List of Threatened Species 2016: e.T10748A115099085. https://dx.doi.org/10.2305/IUCN.UK.2016-3.RLTS.T10748A22232321.en.
Caravaggi, A., P. B. Banks, A. C. Burton, C. M. Finlay, P. M. Haswell, M. W. Hayward, M. J. Rowcliffe and M. D. Wood (2017), ‘A review of camera trapping for conservation behaviour research’, Remote Sensing in Ecology and Conservation, 3 (3), 109–22. https://doi.org/10.1002/rse2.48
Chakraborty, U. (2020), ‘Effects of different phases of the lunar month on living organisms’, Biological Rhythm Research, 51 (2), 254–82. https://doi.org/10.1080/09291016.2018.1526502
Clark, F. E. (2017), ‘Cognitive enrichment and welfare: Current approaches and future directions’. Animal Behavior and Cognition, 4 (1), 52–71. https://doi.org/10.12966/abc.05.02.2017
Clark, F. E. (2018), Competence and agency as novel measures of zoo animal welfare. Measuring Behavior 2018. 171- 172 https://www.researchgate.net/profile/Maurizio-Mauri/publication/328163427_Assessment_of_Storefront_Displays_with_a_Multidisciplinary_Approach_based_on_Neuromarketing_and_Antropological_Marketing/links/5bbc74a4a6fdcc9552dcb5e1/Assessment-of-Storefront-Displays-with-a-Multidisciplinary-Approach-based-on-Neuromarketing-and-Antropological-Marketing.pdf
Clarke, A. and H. O Pörtner (2010), ‘Temperature, metabolic power and the evolution of endothermy’, Biological Reviews, 85 (4), 703–27. https://doi.org/10.1111/j.1469-185X.2010.00122.x
Coppola, F. and A. Felicioli (2021), ‘Reproductive behaviour in free-ranging crested porcupine Hystrix cristata L., 1758’, Scientific Reports, 11 (1), 1–9. https://doi.org/10.1038/s41598-021-99819-3
Coppola, F., G. Vecchio and A. Felicioli (2019), ‘Diurnal motor activity and “sunbathing” behaviour in crested porcupine (Hystrix cristata L., 1758)’, Scientific Reports, 9 (1), 1–8. https://doi.org/10.1038/s41598-019-50784-y
Corsini, M. T., S. Lovari and S. Sonnino (1995), ‘Temporal activity patterns of crested porcupines Hystrix cristata’, Journal of Zoology, 236(1), 43–54. https://doi.org/10.1111/j.1469-7998.1995.tb01783.x
Dagger, T., M. Grützke, M. Reichert, J. Haetge, S. Nowak, M. Winter and F. M. Schappacher (2017), ‘Investigation of lithium ion battery electrolytes containing flame retardants in combination with the film forming electrolyte additives vinylene carbonate, vinyl ethylene carbonate and fluoroethylene carbonate’, Journal of Power Sources, 372, 276–85. https://doi.org/10.1016/j.jpowsour.2017.10.058
Felicioli, A., A. Grazzini and L. Santini (1997), ‘The mounting and copulation behaviour of the crested porcupine Hystrix cristata’, Italian Journal of Zoology, 64 (2), 155–61. https://doi.org/10.1080/11250009709356189
Fernandez-Duque, E. (2003), ‘Influences of moonlight, ambient temperature, and food availability on the diurnal and nocturnal activity of owl monkeys (Aotus azarai)’, Behavioral Ecology and Sociobiology, 54, 431–40. https://doi.org/10.1007/s00265-003-0637-9
Fernandez, E. J., M. A. Tamborski, S. R. Pickens and W. Timberlake (2009), ‘Animal–visitor interactions in the modern zoo: Conflicts and interventions’, Applied Animal Behaviour Science, 120 (1–2), 1–8. https://doi.org/10.1016/j.applanim.2009.06.002
Fleming, P., P. Meek, G. Ballard, P. Banks, A. Claridge, J. Sanderson and D. Swann (Eds.) (2014), Camera Trapping: Wildlife Management and Research. Collingwood, Aus: Csiro Publishing.
Gandia, K. M. (2023), ‘Incorporating circadian and circannual rhythms into welfare assessments of zoo-housed animals’, available at http://hdl.handle.net/1893/36017
Gandia, K. M., Kessler, S. E. and Buchanan-Smith, H. M. (2023), ‘Latitudinal and zoo specific zeitgebers influence circadian and circannual rhythmicity of behavior in captive giant pandas (Ailuropoda melanoleuca)’, Frontiers in Psychology, 14, 1188566. https://doi.org/10.3389/fpsyg.2023.1188566
Gerkema, M. P., W. I. Davies, R. G. Foster, M. Menaker and R. A. Hut (2013), ‘The nocturnal bottleneck and the evolution of activity patterns in mammals’, Proceedings of the Royal Society B: Biological Sciences, 280 (1765), 20130508. https://doi.org/10.1098/rspb.2013.0508
Gilby, I. C., A. A. Pokempner and R. W. Wrangham (2010), ‘A direct comparison of scan and focal sampling methods for measuring wild chimpanzee feeding behaviour’, Folia Primatologica, 81 (5), 254–64. https://doi.org/10.1159/000322354
Giné, G., J. Duarte, T. Motta and D. Faria (2011), ‘Activity, movement and secretive behavior of a threatened arboreal folivore, the thin‐spined porcupine, in the Atlantic forest of southern Bahia, Brazil’, Journal Of Zoology, 286 (2), 131–39. https://doi.org/10.1111/j.1469-7998.2011.00855.x
Hagen, K. B., S. Hammer, S. Frei, S. Ortmann, R. Głogowski and M. Kreuzer (2019), ‘Digestive physiology, resting metabolism and methane production of captive Indian crested porcupine (Hystrix indica)’, Journal of Animal and Feed Sciences, 28, 69–77. https://doi.org/10.22358/jafs/102741/2019
Hall, M. I., J. M. Kamilar and E. C. Kirk (2012), ‘Eye shape and the nocturnal bottleneck of mammals’, Proceedings of the Royal Society B: Biological Sciences, 279 (1749), 4962–68. https://doi.org/10.1098/rspb.2012.2258
Halle, S. (2000), ‘Ecological relevance of daily activity patterns’, in Activity Patterns in Small Mammals (pp. 67–90), Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-18264-8_5
Horton, N. J. and K. Kleinman (2015), Using R and RStudio for Data Management, Statistical Analysis, and Graphics, London: CRC Press.
Kalueff, A. V., A. M. Stewart, C. Song, K. C. Berridge, A. M. Graybiel and J. C. Fentress (2016), ‘Neurobiology of rodent self-grooming and its value for translational neuroscience’, Nature Reviews Neuroscience, 17 (1), 45–59. https://doi.org/10.1038/nrn.2015.8
Kalueff, A. V. and P. Tuohimaa (2004), ‘Grooming analysis algorithm for neurobehavioural stress research’, Brain Research Protocols, 13 (3), 151–58. https://doi.org/10.1016/j.brainresprot.2004.04.002
Kalueff, A. V. and P. Tuohimaa (2005), ‘Mouse grooming microstructure is a reliable anxiety marker bidirectionally sensitive to GABAergic drugs’, European Journal of Pharmacology, 508 (1–3), 147–53. https://doi.org/10.1016/j.ejphar.2004.11.054
Kronfeld-Schor, N., D. Dominoni, H. De la Iglesia, O. Levy, E. D. Herzog, T. Dayan and C. Helfrich-Forster (2013), ‘Chronobiology by moonlight’, Proceedings of the Royal Society B: Biological Sciences, 280 (1765), 20123088. https://doi.org/10.1098/rspb.2012.3088
Law, G. and A. Reid (2010), ‘Enriching the lives of bears in zoos’, International zoo Yearbook, 44 (1), 65–74. https://doi.org/10.1111/j.1748-1090.2009.00096.x
Lillegraven, J. A., Z. Kielan-Jaworowska and W. A. Clemens (Eds.), (1979), Mesozoic Mammals: The First Two-Thirds of Mammalian History. pp. 1–6. Berkeley, CA: University of California Press.
Lovegrove, B. G. (2019) ‘Obligatory nocturnalism in triassic archaic mammals: preservation of sperm quality?’, Physiological and Biochemical Zoology, 92 (6) DOI: 10.1086/705440
Margulis, S. W., C. Hoyos and M. Anderson (2003), ‘Effect of felid activity on zoo visitor interest’, Zoo Biology: Published in affiliation with the American Zoo and Aquarium Association, 22 (6), 587–99. https://doi.org/10.1002/zoo.10115
Mayoral, O., J. Solbes, J. Cantó and T. Pina (2020), ‘What has been thought and taught on the lunar influence on plants in agriculture? perspective from physics and biology’, Agronomy, 10 (7), 955. https://doi.org/10.3390/agronomy10070955
Monterroso, P., P. C. Alves and P. Ferreras (2013), ‘Catch me if you can: diel activity patterns of mammalian prey and predators’, Ethology, 119 (12), 1044–56. https://doi.org/10.1111/eth.12156
Mori, E., S. Lovari, A. Sforzi, G. Romeo, C. Pisani, A. Massolo and L. Fattorini (2014), ‘Patterns of spatial overlap in a monogamous large rodent, the crested porcupine’, Behavioural Processes, 107, 112–18. https://doi.org/10.1016/j.beproc.2014.08.012
Mori, E. and C. Ferrari (2021), ‘Inter-individual behavioural variation in the crested porcupine’, Mammalia, 85 (3), 269–72. https://doi.org/10.1515/mammalia-2020-0104
Moyaho, A. and J. Valencia (2002), ‘Grooming and yawning trace adjustment to unfamiliar environments in laboratory Sprague-Dawley rats (Rattus norvegicus)’, Journal of Comparative Psychology, 116 (3), 263. https://doi.org/10.1037/0735-7036.116.3.263
Mukherjee, A., H. Kumara and S. Bhupathy (2018), ‘Environmental determinants of activity variation of an overlooked burrowing rodent: the Indian crested porcupine’, Mammalia, 82 (5), 449-459. https://doi.org/10.1515/mammalia-2017-0124
O'Connell, A. F., J. D. Nichols and K. U. Karanth (eds), (2011), Camera Traps in Animal Ecology: Methods and Analyses, New York: Springer. https://doi.org/10.1007/978-4-431-99495-4
Nishimura, T. and M. Fukushima (2009), ‘Why animals respond to the full moon: magnetic hypothesis’, Bioscience Hypotheses, 2 (6), 399–401. https://doi.org/10.1016/j.bihy.2009.06.006
Nogueira, S. S., L. G. Bernardi and S. L. Nogueira-Filho (2004), ‘A note on comparative enclosure facility usage by wild and captive-born capybaras (Hydrochoerus hydrochaeris)’, Applied Animal Behaviour Science, 89 (1–2), 139–43. https://doi.org/10.1016/j.applanim.2004.04.007
Packer, C., A. Swanson, D. Ikanda and H. Kushnir (2011), ‘Fear of darkness, the full moon and the nocturnal ecology of African lions’, PloS one, 6 (7), e22285. https://doi.org/10.1371/journal.pone.0022285
Palacín, M. R. and A. de Guibert (2016), ‘Why do batteries fail?’, Science, 351 (6273), 1253292. https://doi.org/10.1126/science.1253292
Palmer, M. S., J. Fieberg, A. Swanson, M. Kosmala and C. Packer (2017), ‘A ‘dynamic ‘landscape of fear: prey responses to spatiotemporal variations in predation risk across the lunar cycle’, Ecology letters, 20 (11), 1364–73. https://doi.org/10.1111/ele.12832
Pomerantz, O., S. Meiri and J. Terkel (2013), ‘Socio-ecological factors correlate with levels of stereotypic behavior in zoo-housed primates’, Behavioural Processes, 98, 85–91. https://doi.org/10.1016/j.beproc.2013.05.005
Prugh, L. R. and C. D. Golden (2014), ‘Does moonlight increase predation risk? Meta‐analysis reveals divergent responses of nocturnal mammals to lunar cycles’, Journal of Animal Ecology, 83 (2), 504–14. https://doi.org/10.1111/1365-2656.12148
Ramírez-Chaves, H.E., C. Romero-Ríos, J.J. Henao-Osorio, J.P. Franco-Herrera and B.R. Ramírez-Padilla, (2020), ‘Notes on the natural history of the stump-tailed porcupine, Coendou rufescens (Rodentia, Erethizontidae), in Colombia’, Neotrop. Biol. Conserv. 15, 471–78. https://doi.org/10.3897/neotropical.15.e56926
Rode-Margono, E. J. and K. A. I. Nekaris, (2014), ‘Impact of climate and moonlight on a venomous mammal, the Javan slow loris (Nycticebus javanicus Geoffroy, 1812)’, Contributions to Zoology, 83 (4), 217–25.https://doi.org/10.1163/18759866-08304001
Rose, P. and L. Riley (2019), ‘The use of qualitative behavioural assessment to zoo welfare measurement and animal husbandry change’, Journal of Zoo and Aquarium Research, 7 (4), 150–61. https://doi.org/10.19227/jzar.v7i4.423
Roze, U. (2014), Porcupines. Baltimore, MD: Johns Hopkins University Press.
RStudio Team. (2019), RStudio: Integrated development for R. Boston, MA: RStudio Inc. available at: https://www.rstudio.com/
Savenije, B., Strubbe, J., & Ritshes-Hoitinga, M. (2010). Nutrition, feeding and animal welfare. The Care and Management of Laboratory and Other Research Animals, 183-193.
Secretary of State’s Standards of Modern Zoo Practice. (2012). Available at https://assets.publishing.service.gov.uk/media/5a78ce01ed915d042206578f/standards-of-zoo-practice.pdf (Accessed 28 August 2024)
Sherwen, S. L. and P. H. Hemsworth (2019), ‘The visitor effect on zoo animals: Implications and opportunities for zoo animal welfare’, Animals, 9 (6), 366. https://doi.org/10.3390/ani9060366
Smolinsky, A. N., C. L. Bergner, J. L. LaPorte and A. V. Kalueff (2009), ‘Analysis of grooming behavior and its utility in studying animal stress, anxiety, and depression’, in T. Gould (ed) Mood and Anxiety Related Phenotypes in Mice: Characterization using behavioral tests, Neuromethods, 42, 21–36. https://doi.org/10.1007/978-1-60761-303-9_2
Torres, C. R. and J. A. Clarke (2018), ‘Nocturnal giants: evolution of the sensory ecology in elephant birds and other palaeognaths inferred from digital brain reconstructions’, Proceedings of the Royal Society B, 285 (1890), 20181540. https://doi.org/10.1098/rspb.2018.1540
Van Aarde, R. J. (1987), ‘Reproduction in the Cape porcupine Hystrix africaeaustralis: An ecological perspective’, South African Journal of Science, 83 (10), 605. https://hdl.handle.net/10520/AJA00382353_5404
Wilson, D. E. and D. M. Reeder (Eds.) (2005), Mammal Species of the World: A Taxonomic and Geographic Reference (Vol. 1), JHU press.
Yarotski, Y. (2022), MoonХ (Version 3.2.1) [Mobile App]. App Store - Apple (UK), available at https://apps.apple.com/gb/app/moon-phase-calander-moonx/id1454249438
Zoo Licensing Act. (1981). Available at https://www.legislation.gov.uk/ukpga/1981/37 (Accessed: 28 August 2024)
Abiotic environmental cue: Non-living factors that trigger changes in organisms and ecosystems.
Allo-grooming: Mutual grooming involving licking the tail, licking the head or the neck of another con specific (Felicioli et al., 1997).
Cathemeral: Activity pattern where an organism is active at anytime of day or night, activity is based on environmental settings.
Circadian: Biological processes that occur over a 24-hour period.
Circannual: A biological process that recurs annually.
Crepuscular: Activity pattern where an organism is active during dusk and dawn.
Diurnal: Activity pattern where an organism is active during the day.
Ectothermic: When an organism is reliant on external sources of heat to maintain body temperature.
Endothermic: When an organism can produce heat internally.
Nocturnality: Activity pattern where an organism is active during the night.
Non-nocturnal diel cycles: Any activity patterns displayed by an organism outside of night.
Non-Parametric: A form of statistical analysis that makes marginal assumptions about the underlying distribution of the data being analyzed.
Photopigment evolution: Unpredictable pigments that experience a chemical change when they absorb light. In different organisms, these pigments have evolved to react differently to light in reaction to different diel cycles.
Stepwise deletion: Stepwise deletion or regression removes the weakest correlated variable.
Trophic co-evolution of behaviours: When the feeding behaviours of two different organisms evolve in response to one another.
To cite this paper please use the following details: Maudsley, H. (2024), 'The Effect of the Lunar Cycle on Nocturnal Behaviour in Captive Cape Porcupine (Hystrix africaeaustralis)’, Reinvention: an International Journal of Undergraduate Research, Volume 17, Issue S1, https://reinventionjournal.org/article/view/1397. Date accessed [insert date]. If you cite this article or use it in any teaching or other related activities please let us know by e-mailing us at Reinventionjournal@warwick.ac.uk.